Stoichiometry - clear & simple (with practice problems) - Chemistry Playlist
Summary
TLDRThe video script offers an engaging and comprehensive guide to understanding stoichiometry, emphasizing its simplicity by breaking it down into four basic problem patterns. It compares stoichiometry to baking, highlighting the importance of balancing chemical equations and calculating mole ratios. The script also differentiates between ideal and limiting reactant stoichiometry, providing clear examples and methodologies for solving problems with moles and mass conversions. It encourages viewers to practice these concepts to master stoichiometry, promising ease in solving complex chemistry problems.
Takeaways
- 📚 Stoichiometry is a fundamental concept in chemistry that involves the quantitative relationships between reactants and products in chemical reactions.
- 🔍 The speaker emphasizes that understanding only four types of stoichiometry problems can significantly simplify the learning process: mole to mole, mole to mass, mass to mole, and mass to mass.
- 🧐 The transcript uses the analogy of baking to explain stoichiometry, highlighting that it's about measuring ingredients to achieve a desired product, similar to balancing chemical equations.
- 🔢 Balancing chemical equations is a crucial first step in stoichiometry, which involves ensuring that the number of atoms for each element is the same on both sides of the equation.
- 📉 The video script discusses the difference between ideal stoichiometry, where reactions are assumed to have perfect conditions, and limiting reactant stoichiometry, which is more realistic and accounts for reactions where one reactant is depleted first.
- 📝 The speaker introduces five key skills needed to master stoichiometry: balancing chemical equations, determining stoichiometric coefficients, understanding mole ratios, distinguishing between ideal and limiting reactant stoichiometry, and solving a variety of stoichiometry problems.
- 🔄 The concept of mole ratios is explained, which involves the relationships between different substances in a balanced chemical equation, allowing for the calculation of reactant and product amounts.
- 📉 The script uses the example of aerobic metabolism of butyric acid to illustrate how to balance a chemical equation and calculate stoichiometric coefficients.
- 📚 The importance of understanding significant figures in stoichiometry calculations is highlighted, ensuring that the answer reflects the precision of the given data.
- 📈 The transcript outlines a step-by-step methodology for solving stoichiometry problems using dimensional analysis, which involves setting up conversion factors and canceling units to find the desired quantity.
- 🔑 The speaker provides practice questions related to stoichiometry, encouraging viewers to apply the concepts learned in the script to solve them, thus reinforcing the learning process.
Q & A
What is the meaning of the term 'Stoichiometry'?
-Stoichiometry refers to the quantitative relationships between reactants and products in a chemical reaction. The term comes from 'stoichio' meaning element and 'metri' meaning measurement.
Why is it important to balance chemical equations before performing stoichiometric calculations?
-Balancing chemical equations is crucial because it ensures that the number of atoms for each element is the same on both sides of the equation, allowing for accurate stoichiometric calculations.
What are the four basic patterns of stoichiometry problems mentioned in the script?
-The four basic patterns of stoichiometry problems are mole to mole, mole to mass, mass to mole, and mass to mass.
What is the difference between ideal stoichiometry and limiting reactant stoichiometry?
-Ideal stoichiometry assumes that there is an unlimited supply of reactants and no loss, resulting in 100% yield. Limiting reactant stoichiometry, on the other hand, takes into account that one of the reactants may run out before the reaction is complete, limiting the amount of product formed.
How many significant figures should be used when reporting the number of moles of calcium in the average human body, according to the script?
-The script suggests using four significant figures when reporting the number of moles of calcium in the average human body.
What is the concept of 'mole ratios' in stoichiometry?
-Mole ratios in stoichiometry refer to the relationships between the amounts of reactants and products in a balanced chemical equation, allowing for the conversion between different quantities of substances involved in a reaction.
What is the correct name for the substance 'CoCl2.6H2O' as discussed in the script?
-The correct name for 'CoCl2.6H2O' is Cobalt(II) chloride hexahydrate, indicating a compound with Cobalt in the +2 oxidation state and six water molecules of hydration.
What is the philosophy of Stoicism, and how does it relate to the concept of stoichiometry?
-The philosophy of Stoicism is an ancient school of philosophy that promotes the idea of accepting whatever happens in life. In the script, it is used as a metaphor to emphasize the importance of keeping things simple and basic in stoichiometry, similar to the stoic approach to life.
How does the script compare stoichiometry to baking a cake?
-The script compares stoichiometry to baking a cake by explaining that just as you need specific amounts of ingredients to bake a cake, you need specific ratios of reactants to produce a certain amount of products in a chemical reaction.
What is the purpose of the 'five steps to surviving chemistry' methodology mentioned in the script?
-The 'five steps to surviving chemistry' methodology is a systematic approach to solving stoichiometry problems. It involves starting with the given information, using dimensional analysis to set up conversion factors, repeating the process as needed for multiple conversions, performing the math, and reporting the answer with the correct units.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

BERPIKIR KOMPUTASIONAL - 4 FONDASI BK

IPA SMA Kelas 10 - Reaksi Kimia | GIA Academy

What is Pseudocode Explained | How to Write Pseudocode Algorithm | Examples, Benefits & Steps

6 Levels of Dialogue Every Writer MUST Master

Conditional Sentence Dalam Bahasa Inggris
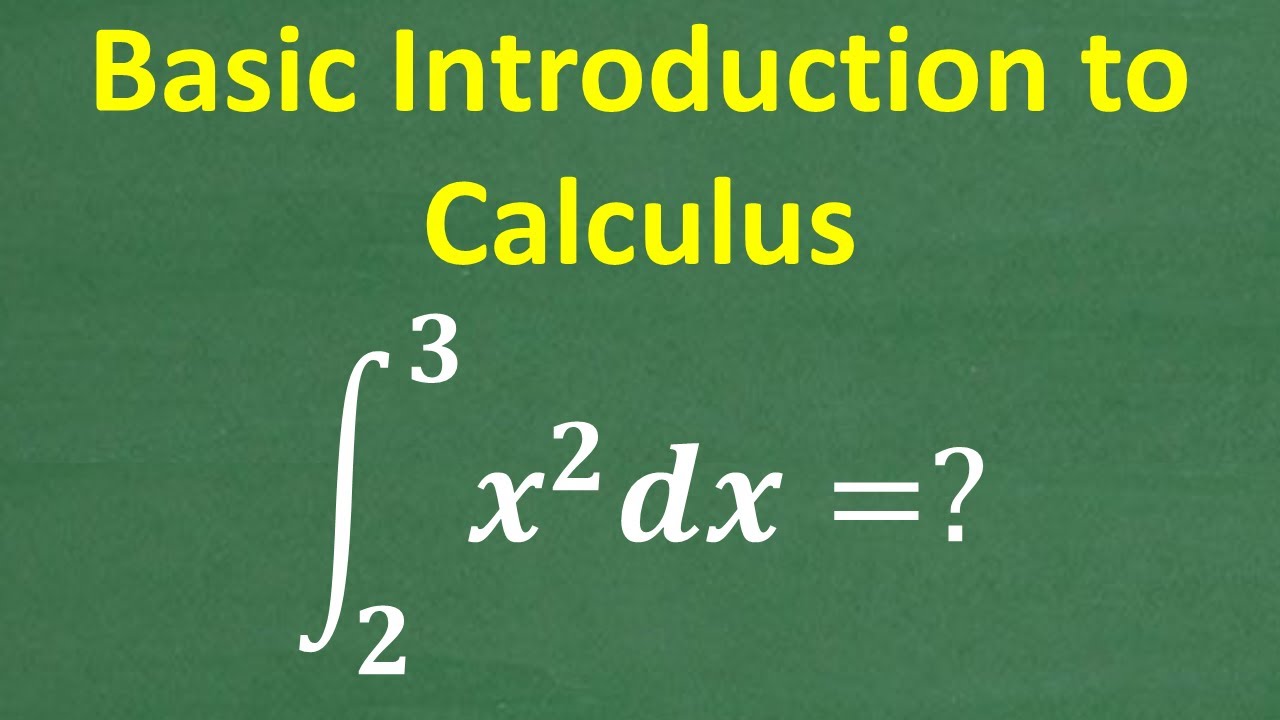
BASIC Calculus – Understand Why Calculus is so POWERFUL!
5.0 / 5 (0 votes)