Constante de Equilíbrio: Kp e Kc
Summary
TLDRIn today's lesson, we learn how to calculate the equilibrium constants, KP and KC, for reversible reactions. The script explains the relationship between the rate laws of elementary reactions and the constants of equilibrium, as well as the significance of concentration and pressure in these calculations. It highlights that substances in solid or liquid states are excluded from equilibrium expressions, and only gas and aqueous substances are considered. Additionally, the lesson outlines how to express equilibrium constants using concentration or partial pressure, with examples to illustrate the process.
Takeaways
- 😀 The equilibrium constants, KP and KC, are used to describe reversible reactions.
- 😀 To find the rate law for an elementary reaction, multiply the reaction constant by the concentration of each reactant raised to its stoichiometric coefficient.
- 😀 A reversible reaction has two directions: direct (reactants to products) and inverse (products to reactants).
- 😀 The rate of the direct reaction is proportional to the concentrations of the reactants, while the rate of the inverse reaction depends on the concentrations of the products.
- 😀 At equilibrium, the rate of the direct reaction equals the rate of the inverse reaction, making the system stable.
- 😀 The equilibrium constant KC is the ratio of the product of the concentrations of the products raised to their coefficients over the product of the concentrations of the reactants raised to their coefficients.
- 😀 When concentration is expressed in mol/L, the equilibrium constant is denoted KC; when expressed in terms of partial pressures of gases, it is denoted KP.
- 😀 Solids and liquids are not included in the equilibrium expression for KC or KP, only gases or aqueous substances are considered.
- 😀 For example, the equilibrium expression for a reaction like FeSO4 dissociating into H2 and H2SO4 would exclude solid Fe.
- 😀 To find KP, use the partial pressures of the gases in the reaction and raise them to their respective coefficients.
- 😀 In the next lesson, the relationship between equilibrium constants and reaction yield will be discussed, along with the effect of temperature on equilibrium.
Q & A
What is the main topic of this lesson?
-The main topic of the lesson is understanding how to find the equilibrium constants KP and KC for reversible chemical reactions.
What is the general method for determining the rate law of an elementary reaction?
-To determine the rate law of an elementary reaction, we multiply the characteristic rate constant of the reaction by the concentration of each reactant raised to the power of their respective stoichiometric coefficients.
How is the rate of a reaction expressed in terms of reactants A and B?
-The rate of the reaction is expressed as the rate constant times the concentration of reactant A raised to its stoichiometric coefficient times the concentration of reactant B raised to its stoichiometric coefficient.
What does it mean when a reaction is reversible?
-A reversible reaction has both a forward reaction, where reactants turn into products, and a reverse reaction, where products turn back into reactants.
What happens when a reversible reaction reaches equilibrium?
-When a reversible reaction reaches equilibrium, the rate of the forward reaction equals the rate of the reverse reaction, meaning no net change in the concentrations of reactants and products.
What is the relationship between the rate constants of the forward and reverse reactions?
-The ratio of the rate constant of the forward reaction to that of the reverse reaction is called KC, the equilibrium constant, which is used to express the equilibrium state of the reaction.
What factors are considered when calculating the equilibrium constant KC?
-When calculating KC, only the concentrations of aqueous and gaseous substances are considered, with the concentrations of solid and liquid substances excluded from the equilibrium expression.
How does the equilibrium constant KP differ from KC?
-KP is used when the concentrations of the reactants and products are expressed in terms of partial pressures of gaseous substances, whereas KC uses concentrations in mol/L.
Why are solids and liquids excluded from equilibrium expressions?
-Solids and liquids are excluded from equilibrium expressions because their concentrations do not change appreciably during the reaction, making them irrelevant to the equilibrium calculation.
How do you calculate KC for the reaction involving FeSO4, H2, and H2SO4?
-To calculate KC for this reaction, we write the expression as KC = [FeSO4] * [H2] / [H2SO4], excluding the concentration of Fe as it is a solid.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
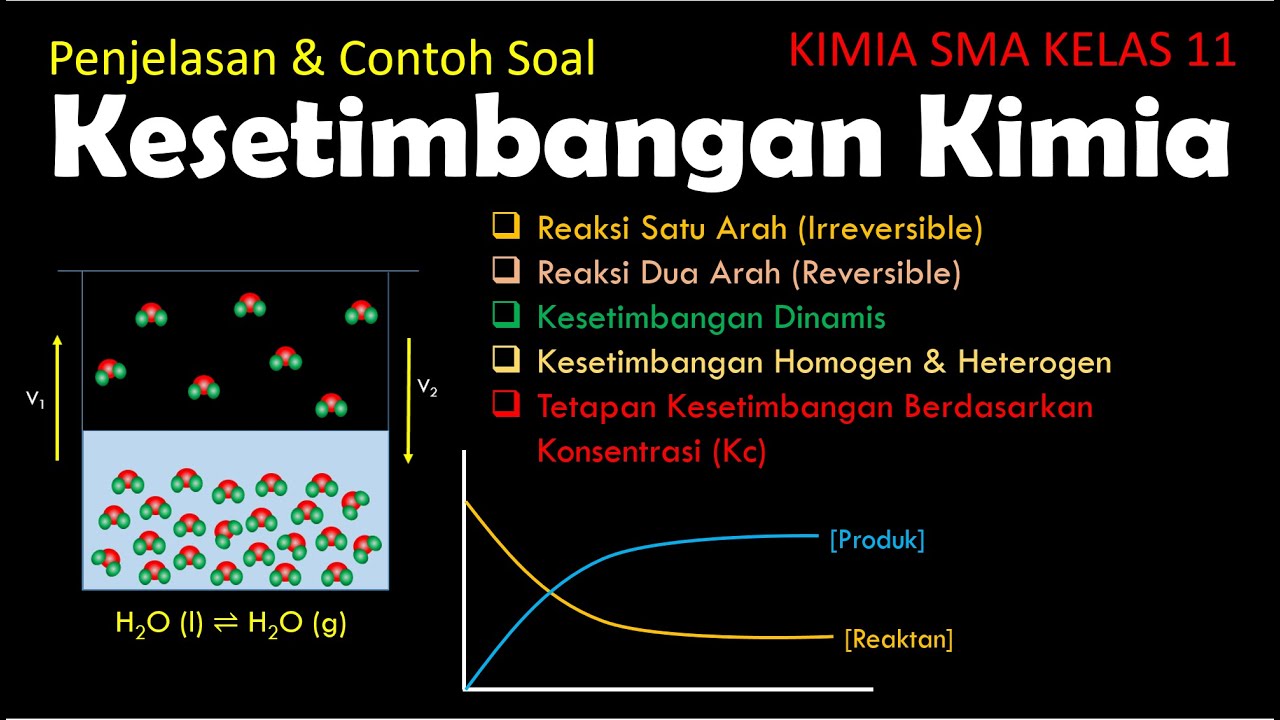
KESETIMBANGAN KIMIA ( KIMIA SMA KELAS 11 )

Interpretação da Constante de Equilíbrio

Kesetimbangan Kimia • Part 1: Konsep, Hukum, Tetapan Kesetimbangan Kc dan Kp

Kesetimbangan Kimia| Kimia SMA | Tetty Afianti

PENGERTIAN DAN TETAPAN KESETIMBANGAN (Kc)

Kesetimbangan Kimia • Part 3: Tetapan Kesetimbangan Pada Reaksi Berkaitan / Hukum Hess
5.0 / 5 (0 votes)