Kelarutan dan KSP | Kimia SMA | Tetty Afianti
Summary
TLDRIn this chemistry lesson, the teacher discusses the concepts of solubility and solubility product (Ksp). The lesson covers different types of solutions—unsaturated, saturated, and supersaturated—illustrating the process with examples like coffee and water. The teacher then explains the solubility calculations for binary, ternary, and quaternary solutions, each with distinct ion compositions. Various equations for determining solubility and Ksp are introduced. The video also includes practical examples and problems related to NaCl, CaF2, CaSO4, and others. The session wraps up with the relationship between Ksp and pH, emphasizing the importance of understanding solubility in chemistry.
Takeaways
- 😀 Before starting any lesson, it's important to say 'Bismillahirrohmanirrohim' to seek ease in understanding the material.
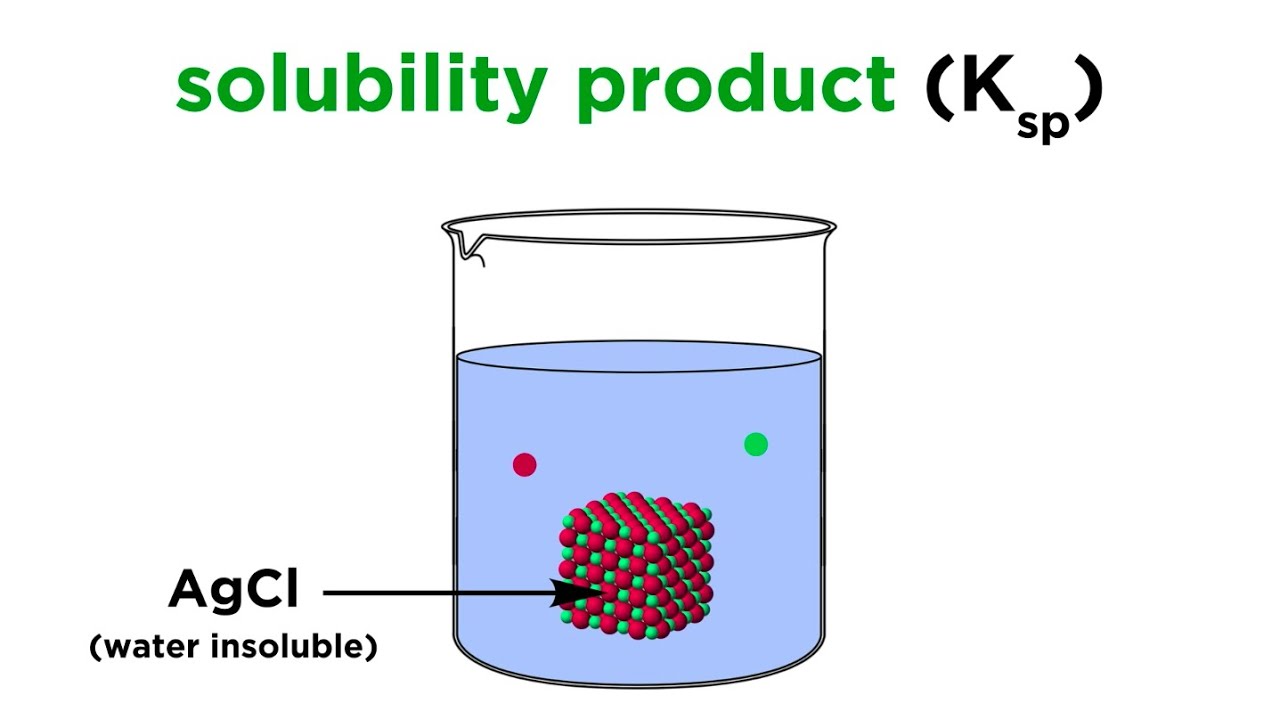
- 😀 The lesson covers solubility and solubility product (Ksp), which are essential concepts in chemistry.
- 😀 A solution consists of two components: the solvent (e.g., pure water) and the solute (e.g., coffee powder).
- 😀 A solution can be in three states: unsaturated, saturated, and supersaturated, depending on the solute's ability to dissolve.
- 😀 In unsaturated solutions, all the solute dissolves easily, while in saturated solutions, no more solute can dissolve.
- 😀 Supersaturated solutions occur when more solute is added than the solvent can hold, leading to excess solute that does not dissolve.
- 😀 Binary solutions consist of two ions, like NaCl (one Na+ ion and one Cl- ion), and can be calculated using the formula Ksp = x^2.
- 😀 Ternary solutions consist of three ions, like H2SO4 (two H+ ions and one SO4^2- ion), and their solubility product is calculated using the formula Ksp = 4S^3.
- 😀 Quaternary solutions consist of four ions, such as H3PO4 (three H+ ions and one PO4^3- ion), and are calculated using Ksp = 27S^4.
- 😀 The relationship between Ksp and pH is reciprocal: Ksp can be used to calculate pH, and pH can be used to determine Ksp.
- 😀 Several example problems are included, showing how to calculate solubility and Ksp for various compounds like NaCl, CaSO4, and FeOH2.
Q & A
What is the importance of saying 'Bismillahirrohmanirrohim' before starting the lesson?
-Saying 'Bismillahirrohmanirrohim' is a way to ask for blessings and ease in understanding the lesson, as it is a practice in Islamic culture to seek help from God before engaging in any activity.
What are the components of a solution in chemistry?
-A solution consists of two components: the solvent, which is the substance that dissolves the solute, and the solute, which is the substance that dissolves in the solvent. For example, in coffee, water is the solvent and coffee powder is the solute.
What is the difference between unsaturated, saturated, and supersaturated solutions?
-An unsaturated solution can dissolve more solute, a saturated solution has dissolved all the solute it can at a given temperature, and a supersaturated solution contains more solute than it should, which can lead to crystallization if disturbed.
What is a binary solution and how is it calculated?
-A binary solution contains two ions. An example is NaCl, which dissociates into Na+ and Cl-. The solubility product (Ksp) for binary solutions is calculated using the formula Ksp = x^2, where x is the solubility.
What is a ternary solution and how is its solubility calculated?
-A ternary solution contains three ions. An example is H2SO4, which dissociates into 2 H+ ions and 1 SO4^2-. The solubility product for ternary solutions is calculated using the formula Ksp = 4S^3, where S is the solubility.
How do you calculate the solubility of a quaternary solution?
-A quaternary solution contains four ions. An example is H3PO4, which dissociates into 3 H+ ions and 1 PO4^3- ion. The solubility product is calculated using the formula Ksp = 27S^4, where S is the solubility.
What is the relationship between Ksp and pH?
-Ksp and pH have an inverse relationship. By knowing the Ksp of a solution, you can calculate its pH, and conversely, knowing the pH can help determine the Ksp.
How do you calculate Ksp from a given solubility value?
-To calculate the Ksp from solubility, you use the appropriate formula based on the type of solution (binary, ternary, or quaternary). For example, for a binary solution, Ksp = x^2, where x is the solubility in mol/L.
What is the process to calculate pH for a strong base like CaOH2?
-To calculate the pH for a strong base like CaOH2, first calculate the concentration of OH- ions using the Ksp value, then find the pOH. The pH is then calculated as 14 minus the pOH.
How do you calculate the pH of a solution of H2SO4?
-For H2SO4, a strong acid, you calculate the concentration of H+ ions from the solubility product, and then use the formula pH = -log[H+]. As H2SO4 dissociates completely, the concentration of H+ is directly proportional to its solubility.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Kelarutan dan Hasil Kali Kelarutan (1) | Hubungan S dgn Ksp | Kimia Kelas 11

PEKI4205 Kimia Analitik I - Analisis Gravimetri

HASIL KALI KELARUTAN (KSP) -Simple Konsep- KIMIA 11 (Kursus Online Rp8.000 per BULAN :cek deskripsi)

Kelarutan dan Hasil Kali Kelarutan (Ksp) Kimia Kelas 11 • Part 1: Konsep, Hubungan Kelarutan dan Ksp

Ksp, Prakiraan Pengendapan dan Hubungan Ksp dg pH
Solubility Product Constant (Ksp)
5.0 / 5 (0 votes)