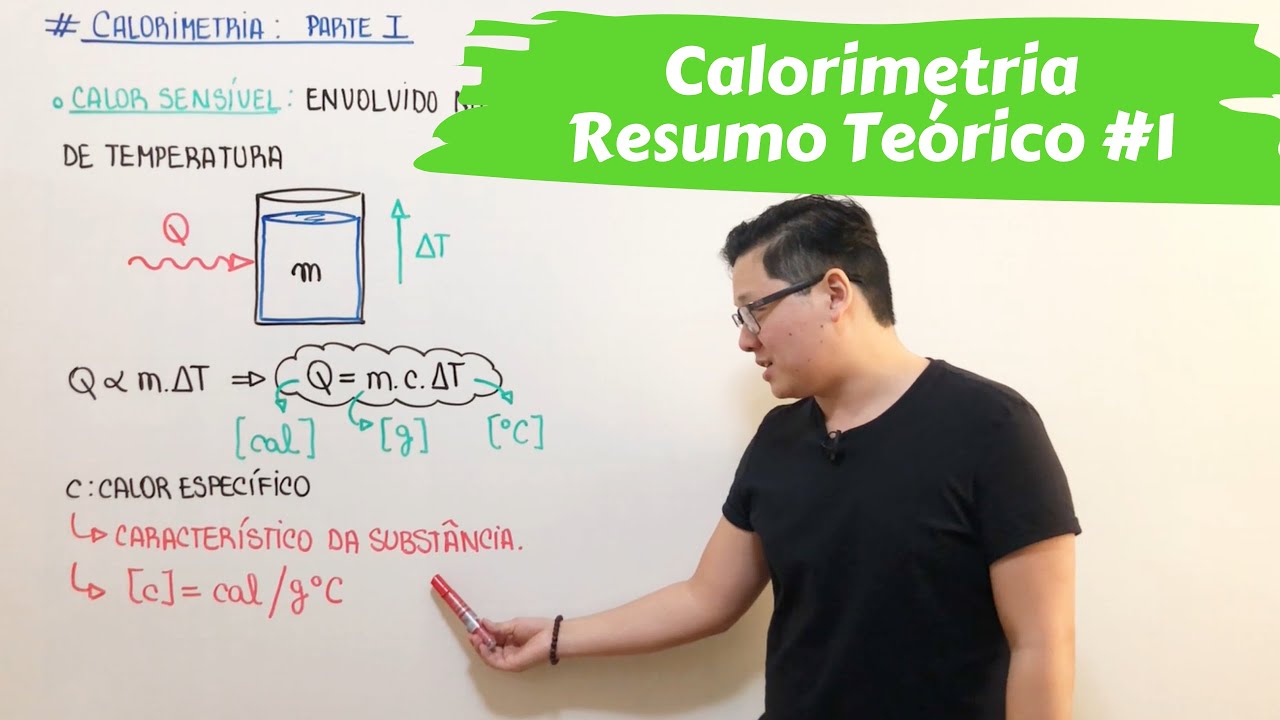
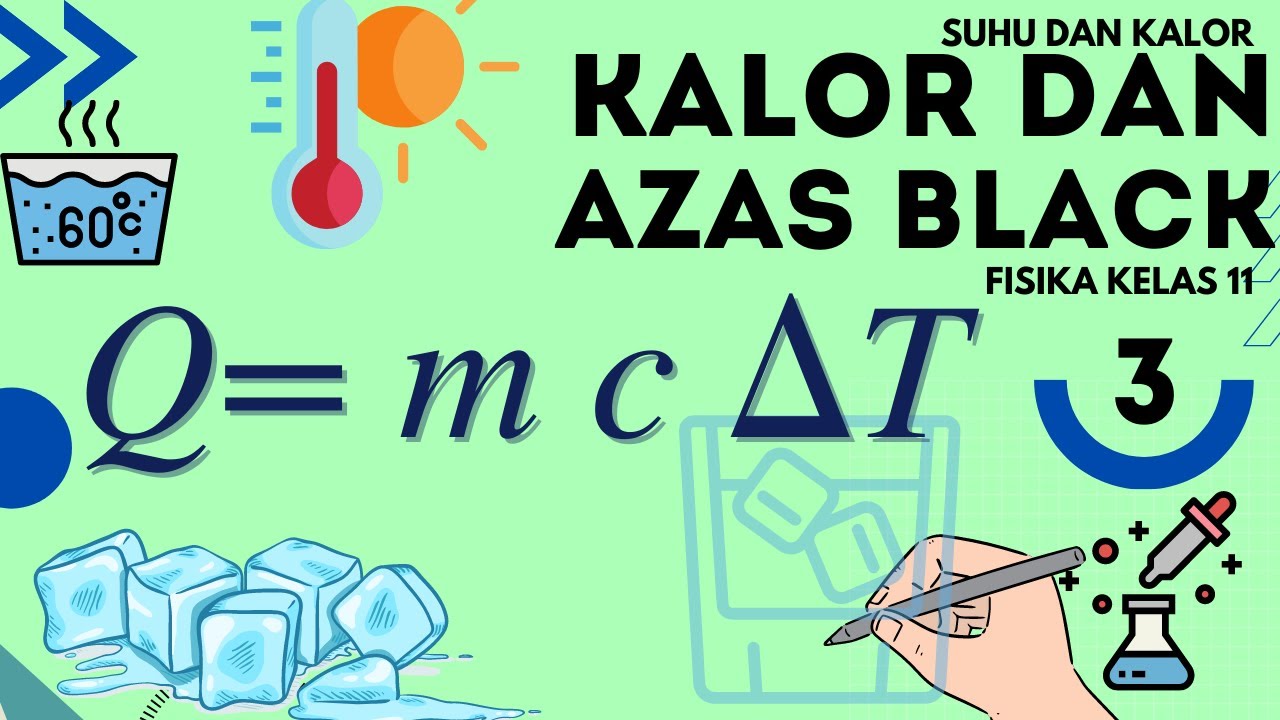Física - Trocas de Calor
Summary
TLDRThis educational video lesson dives into the concept of heat exchange and calorimetry, focusing on the principles of sensible and latent heat. The speaker explains how heat is transferred between substances at different temperatures and how thermal equilibrium is reached in a thermally insulated system. Using an example of mixing two water portions at 80°C and 20°C, the speaker demonstrates how to calculate the final equilibrium temperature through the heat exchange equation. The lesson emphasizes the importance of understanding heat loss and gain, offering clear insights into calorimetry for students learning physics.
Takeaways
- 😀 Heat exchange occurs when two bodies at different temperatures come into contact and transfer heat until they reach thermal equilibrium.
- 😀 A thermally insulated system is designed to minimize heat exchange with the external environment, such as a cooler or thermos.
- 😀 In a thermally insulated system, the heat transferred occurs only between the bodies inside, without any influence from the surroundings.
- 😀 Sensible heat is responsible for temperature changes in a substance, while latent heat is involved in phase changes (e.g., melting, boiling).
- 😀 Heat given off by a body with a higher temperature is considered negative, while heat received by a body with a lower temperature is considered positive.
- 😀 The sum of all the heat exchanged in a thermally insulated system equals zero, meaning heat lost by one body is gained by another.
- 😀 In the given example, mixing 500 g of water at 80°C with 300 g of water at 20°C, the thermal equilibrium temperature was calculated to be approximately 57.5°C.
- 😀 The specific heat of water is assumed to be 1 cal/g°C, which simplifies calculations for heat exchange in water-related problems.
- 😀 Thermal equilibrium is reached when both bodies in contact achieve the same final temperature, as seen in the water mixing example.
- 😀 The final temperature in the example is closer to the temperature of the larger mass of water (80°C), since the larger mass has more heat to transfer.
- 😀 Understanding both sensible and latent heat is crucial for accurately solving calorimetry problems and calculating heat exchanges in thermal systems.
Q & A
What is the primary focus of the lesson discussed in the video?
-The primary focus of the lesson is on heat exchange and calorimetry, particularly the concept of thermal equilibrium between two bodies of different temperatures.
What is the difference between sensible heat and latent heat?
-Sensible heat is responsible for temperature changes without changing the substance's physical state, while latent heat is responsible for the change in the physical state of a substance without changing its temperature.
What is a thermally insulated system?
-A thermally insulated system is one that minimizes heat exchange with the external environment, allowing only heat transfer between the bodies inside the system.
Why is the cooler in the example considered a thermally insulated system?
-The cooler is considered thermally insulated because it aims to reduce heat transfer with the external environment, allowing heat exchange only between the cans and the ice inside.
What happens when two substances with different temperatures are placed in thermal contact?
-When two substances with different temperatures are in thermal contact, heat will flow from the hotter substance to the cooler one until thermal equilibrium is reached, where both substances have the same temperature.
What does the heat exchange equation for thermally insulated systems state?
-The heat exchange equation for thermally insulated systems states that the sum of heat given off must equal the sum of heat received, resulting in a net heat exchange of zero.
How is heat exchange represented in terms of sign convention?
-In the sign convention, heat given off is considered negative (since the body loses heat), and heat received is positive (since the body gains heat).
In the example of mixing water at 80°C and 20°C, what will be the final temperature of the mixture?
-The final temperature of the mixture will be around 57.5°C, which is between the initial temperatures of 80°C and 20°C. This temperature is closer to 80°C because the mass of the hot water is larger.
Why is the final temperature of the water mixture closer to 80°C than to 20°C?
-The final temperature is closer to 80°C because the amount of hot water (500g) is greater than the amount of cold water (300g), so the heat transfer is greater from the hot water.
What additional concept must be understood alongside heat exchange in calorimetry?
-In addition to heat exchange, it is important to understand sensible heat and latent heat, as they are intertwined in the study of calorimetry.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)