Introduction to Infrared Spectroscopy
Summary
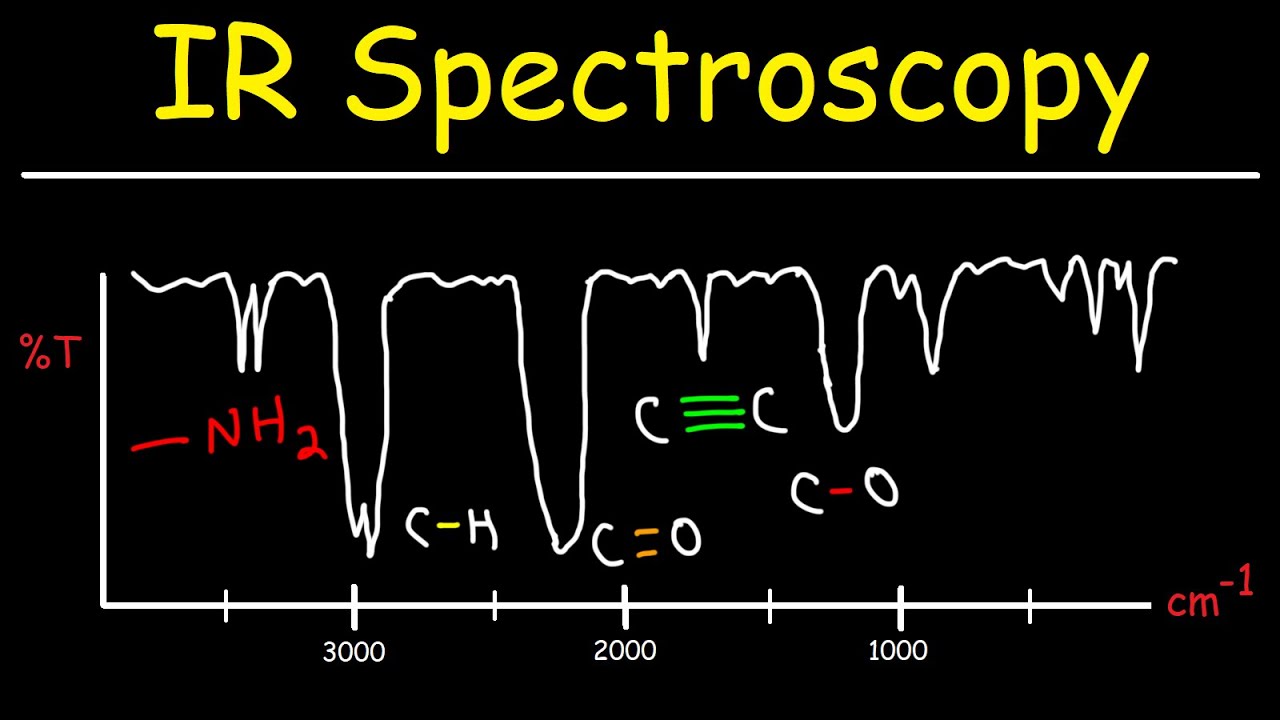
TLDRThis lecture introduces the fundamentals of IR spectroscopy, explaining how different functional groups in molecules vibrate at specific frequencies. The IR machine works by shining light at various frequencies, which are absorbed if they match the vibration frequencies of the functional groups. The resulting absorption bands in the IR spectrum help identify the presence of specific functional groups. The functional group region (1800-4000 cm⁻¹) and fingerprint region are key in molecule identification. The lecture emphasizes the importance of understanding these absorption bands for analyzing molecular structures in organic chemistry.
Takeaways
- 😀 Different functional groups vibrate at distinct frequencies (bend, stretch, wag) in IR spectroscopy.
- 😀 A functional group absorbs light if the frequency of the light matches its vibrational frequency.
- 😀 The IR machine measures the absorption of light by a sample, which helps determine the presence of functional groups.
- 😀 Carbonyl groups (C=O) in molecules like formaldehyde stretch and wag at specific frequencies.
- 😀 The absorption of light by a molecule creates a dip in the IR spectrum, called an absorption band.
- 😀 The X-axis in an IR spectrum typically represents wave numbers, not frequencies, with the functional group region ranging from 4000 to 1800 cm⁻¹.
- 😀 The Y-axis in an IR spectrum represents the percent of transmitted light, indicating how much light passes through the sample.
- 😀 The fingerprint region of an IR spectrum contains unique absorption patterns for different molecules, making it useful for identifying substances.
- 😀 IR spectroscopy helps determine which functional groups are present in a molecule, providing insights into its structure.
- 😀 A chart of known wave numbers for different functional groups helps interpret IR spectra and identify specific molecules.
- 😀 Molecules can vibrate in various ways (e.g., symmetric/asymmetric stretching, scissoring, rocking), each corresponding to different absorption bands in the IR spectrum.
Q & A
What is the main focus of this lecture on IR spectroscopy?
-The main focus of the lecture is to explain the basics of IR spectroscopy, how the IR machine operates, and how it helps identify functional groups in molecules by observing absorption bands.
How does IR spectroscopy help identify functional groups in a molecule?
-IR spectroscopy works by shining light at a sample molecule. If the frequency of the light matches the vibration frequency of a functional group (like stretching, bending, or wagging), the molecule absorbs the light, and the corresponding absorption bands can be analyzed to identify functional groups.
What is a carbonyl group, and how does it behave in IR spectroscopy?
-A carbonyl group is a functional group consisting of a carbon doubly bonded to an oxygen (C=O). In IR spectroscopy, this group stretches and wags at specific frequencies, which can be detected as absorption bands.
Why does the carbonyl group in formaldehyde vibrate at specific frequencies?
-The carbonyl group in formaldehyde vibrates because the bond between carbon and oxygen behaves like a spring, allowing it to stretch, compress, and even wag back and forth at distinct frequencies, such as 2 Hz and 4 Hz.
What happens when light at a frequency that matches a molecule’s vibration is shined on it?
-When light at a frequency that matches the vibration frequency of a molecule (such as stretching, bending, or wagging) is shined on it, the molecule absorbs the light, and this leads to a reduction in the amount of transmitted light detected by the IR machine.
How does the IR spectrometer detect absorption bands?
-The IR spectrometer shines light at varying frequencies onto the molecule. The detector then records the amount of light that passes through the sample. If the molecule absorbs light at certain frequencies, absorption bands appear on the resulting spectrum.
What is the significance of the functional group region on an IR spectrum?
-The functional group region, which ranges from 1800 cm⁻¹ to 4000 cm⁻¹, contains absorption bands that are used to identify specific functional groups in a molecule based on their characteristic vibration frequencies.
Why do IR absorption bands appear at specific wave numbers, and how is this different from frequency?
-Absorption bands appear at specific wave numbers because wave number is inversely related to the frequency of light. While frequency measures cycles per second, wave number measures the number of waves per centimeter, offering a different way of representing the same data.
What is the fingerprint region in an IR spectrum?
-The fingerprint region, which lies below 1800 cm⁻¹, contains absorption bands that are unique to individual molecules. This region can be used to identify molecules because it contains distinct patterns specific to the structure of each compound.
How do different types of molecular vibrations contribute to the IR spectrum?
-Different types of molecular vibrations, such as symmetrical and asymmetrical stretching, scissoring, rocking, twisting, and wagging, each have their corresponding absorption bands in the IR spectrum. These different vibrations can help provide more detailed information about the molecule's structure.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)