Grade 10 Electromagnetic Radiation Physics: Introduction
Summary
TLDRIn this introductory video, Miss Martin explains the basics of electromagnetic radiation, a key concept in physics. She contrasts electromagnetic waves with mechanical waves, highlighting that they do not require a medium to travel. The video covers the electromagnetic spectrum, from high-energy gamma rays to low-energy radio waves, and emphasizes the importance of understanding the order and characteristics of these waves. The dual nature of electromagnetic radiation, exhibiting both wave-like and particle-like behavior, is introduced. Miss Martin also discusses the speed of light, its formula, and the relationship between frequency and wavelength, setting the stage for future lessons and calculations.
Takeaways
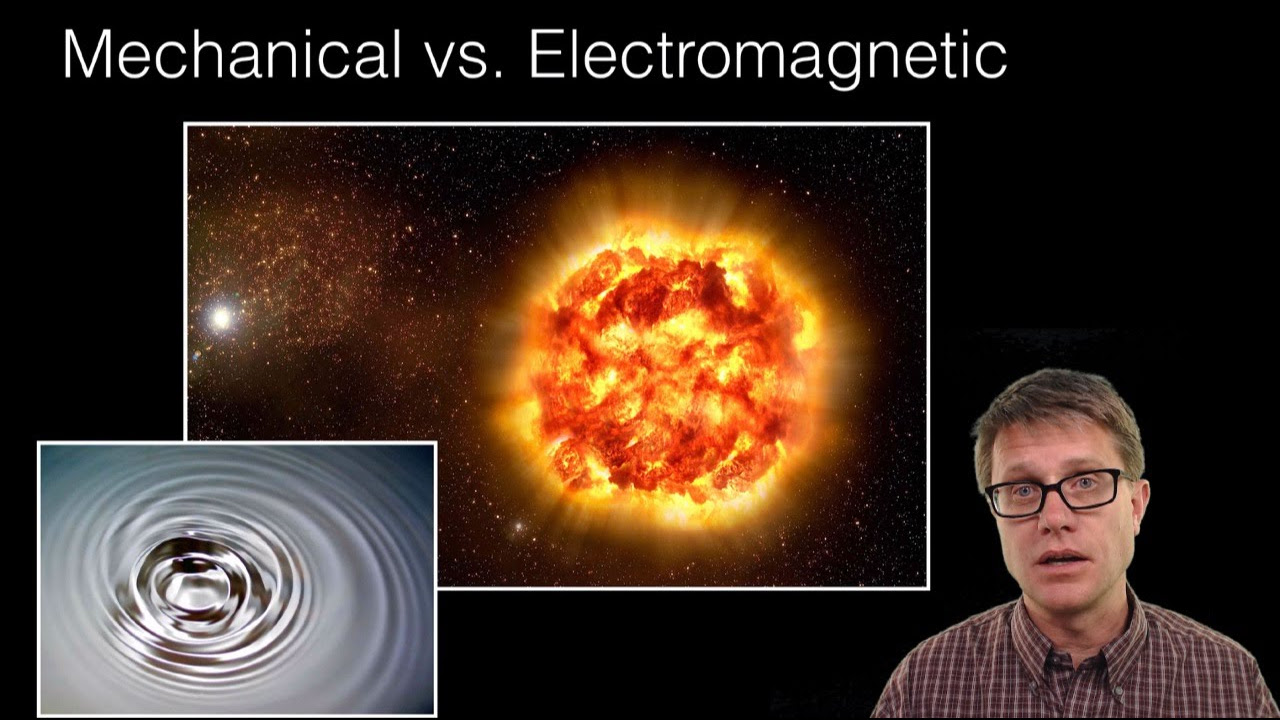
- 😀 Electromagnetic radiation does not require a medium to travel, unlike mechanical waves that need solids, liquids, or gases.
- 😀 The electromagnetic spectrum includes different types of waves, from gamma rays (high frequency, high energy) to radio waves (low frequency, low energy).
- 😀 A helpful acronym to remember the order of the electromagnetic spectrum is 'Raging Martians Invaded Venus Using X-ray Guns' (Radio waves, Microwaves, Infrared, Visible light, Ultraviolet, X-rays, Gamma rays).
- 😀 Electromagnetic radiation consists of varying electric and magnetic fields, with these fields oscillating at right angles to each other, transferring energy.
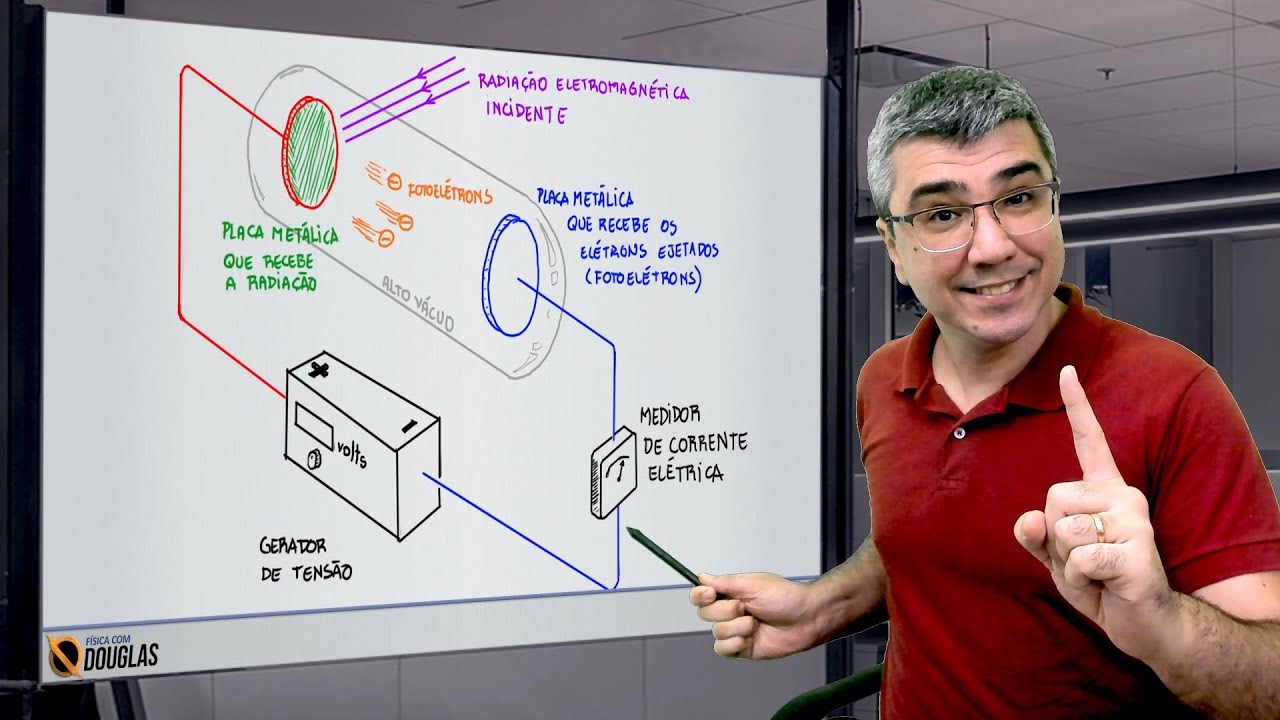
- 😀 Waves exhibit a wave-particle duality, meaning light can behave both as a wave (when passing through a slit) and as a particle (in the photoelectric effect).
- 😀 All electromagnetic radiation travels at the speed of light, denoted as 'c', which is 3 × 10^8 m/s, and this speed is constant in a vacuum.
- 😀 The formula to calculate the speed of an electromagnetic wave is c = frequency × wavelength, where 'c' is the speed of light, frequency is in Hertz, and wavelength is in meters.
- 😀 The frequency and wavelength of waves are inversely proportional: high frequency correlates with short wavelength, and low frequency correlates with long wavelength.
- 😀 The visible light spectrum consists of red, orange, yellow, green, blue, indigo, and violet (ROYGBIV), with red having the longest wavelength and violet having the shortest.
- 😀 It’s essential to know the uses and examples of various types of electromagnetic radiation such as infrared, ultraviolet, X-rays, and microwaves, as they have practical applications in daily life and technology.
Q & A
What is electromagnetic radiation?
-Electromagnetic radiation refers to waves that transmit energy through varying electric and magnetic fields. Unlike mechanical waves, electromagnetic waves do not require a medium to travel through and can propagate through a vacuum.
How does electromagnetic radiation differ from mechanical waves?
-Mechanical waves require a medium (such as air, water, or solids) to propagate and involve the vibration of particles. In contrast, electromagnetic waves do not require a medium and propagate through oscillating electric and magnetic fields.
What is the importance of understanding the electromagnetic spectrum?
-Understanding the electromagnetic spectrum is crucial because it helps classify different types of electromagnetic radiation based on their wavelengths and frequencies. This knowledge allows us to identify various types of waves, from gamma rays to radio waves, and their corresponding uses.
What is the order of the electromagnetic spectrum?
-The electromagnetic spectrum can be remembered using the acronym 'Raging Martians Invaded Venus Using X-ray Guns' or 'So Good Xylophones Use Very Interesting Musical Rhythms.' The order is: Gamma rays, X-rays, Ultraviolet, Visible light, Infrared, Microwaves, and Radio waves.
What is meant by the term 'wave-particle duality'?
-Wave-particle duality refers to the phenomenon where light can sometimes behave like a wave (e.g., when it spreads out after passing through a slit) and sometimes behave like a particle (e.g., when it causes electrons to be ejected from a metal surface in the photoelectric effect).
What is the speed of light, and why is it significant in electromagnetic radiation?
-The speed of light, denoted as 'C', is a constant value of 3 x 10^8 meters per second. It is significant because all types of electromagnetic radiation, regardless of their wavelength or frequency, travel at the same speed in a vacuum.
How can the speed of electromagnetic waves be calculated?
-The speed of electromagnetic waves is calculated using the formula 'C = frequency x wavelength', where 'C' is the speed of light (3 x 10^8 m/s), frequency is measured in Hertz (Hz), and wavelength is measured in meters.
What is the relationship between frequency and wavelength in the electromagnetic spectrum?
-Frequency and wavelength are inversely proportional. This means that as the frequency increases, the wavelength decreases, and vice versa. For example, gamma rays have high frequency and short wavelengths, while radio waves have low frequency and long wavelengths.
What are the uses of different types of electromagnetic radiation?
-Electromagnetic radiation has various practical applications. For example, X-rays are used in medical imaging, microwaves are used in cooking and communications, and infrared radiation is used in thermal imaging. Radio waves are used in communication systems, and ultraviolet radiation has applications in sterilization.
What does it mean that all electromagnetic waves travel at the same speed in a vacuum?
-It means that, regardless of whether the wave is a gamma ray, radio wave, or any other type, the speed of electromagnetic radiation is constant in a vacuum, specifically 3 x 10^8 meters per second. This uniform speed applies to all types of electromagnetic waves.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)