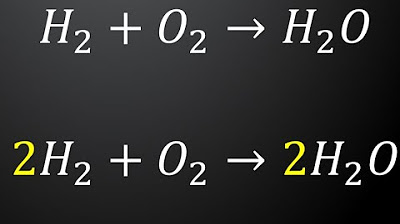Persamaan Reaksi Kimia dan Cara Mudah Menyetarakan Reaksi Kimia
Summary
TLDRThis video explains how to balance chemical equations, starting with understanding the components of a chemical equation, including reactants and products. The process involves writing the unbalanced equation, identifying the state of each substance, and adjusting the coefficients to ensure the equation adheres to the Law of Conservation of Mass. It provides a detailed example of balancing the equation involving aluminum and hydrochloric acid, covering the steps of balancing atoms in a systematic order. The final equation is simplified to whole numbers to ensure the correct stoichiometric ratio is achieved. Watch to learn the essential method for balancing chemical reactions!
Takeaways
- 😀 A chemical reaction equation represents the process of chemical reactions, showing the substances that react and the products formed.
- 😀 A chemical reaction equation consists of two parts: the left side (reactants) and the right side (products).
- 😀 The left side shows the reactants or substances that undergo change during the reaction, while the right side shows the resulting products.
- 😀 The steps to write a balanced chemical equation include: writing the reaction, indicating states of matter using symbols (G for gas, L for liquid, S for solid, AQ for aqueous), and then adding the appropriate coefficients.
- 😀 Coefficients in a reaction indicate the simplest ratio of particles involved, and if not written, the coefficient is assumed to be 1.
- 😀 To balance chemical equations, the law of conservation of mass must be followed: the mass before and after the reaction should remain constant, meaning the number of atoms should be the same on both sides.
- 😀 The balancing process begins by assigning a coefficient of 1 to the most complex compound, followed by balancing atoms step-by-step, starting with cations, anions, hydrogen, and oxygen.
- 😀 Example of a reaction: Al (S) + HCl (AQ) → AlCl3 (AQ) + H2 (G). The process includes setting coefficients for the most complex substance, balancing atoms, and ensuring they match on both sides.
- 😀 If there is a fraction in the coefficient, it should be multiplied by a number to convert it to a whole number for simplicity, ensuring that all coefficients are integers.
- 😀 The final balanced reaction for the example includes coefficients like 2 for HCl and 3 for H2 after the necessary adjustments and simplifications.
- 😀 The video encourages viewers to subscribe and continue following the channel for more educational chemistry content.
Q & A
What is a chemical reaction equation?
-A chemical reaction equation is a formula that represents a chemical process, showing the substances that react and the products formed during the reaction.
What are the two parts of a chemical reaction equation?
-A chemical reaction equation consists of two parts: the left side, which represents the reactants (substances that undergo the reaction), and the right side, which represents the products (the result of the reaction).
What symbols are used to represent the states of substances in a chemical equation?
-In a chemical equation, symbols such as 'G' for gas, 'L' for liquid, 'S' for solid, and 'AQ' for aqueous solution are used to represent the states of substances.
What is the role of coefficients in a chemical reaction equation?
-Coefficients in a chemical reaction equation indicate the simplest ratio of the number of particles involved in the reaction, and they are placed in front of the chemical formulas to balance the equation.
How is the law of conservation of mass related to a chemical reaction equation?
-The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction, meaning the number of atoms before and after the reaction must be the same.
What is the first step in balancing a chemical reaction equation?
-The first step in balancing a chemical reaction equation is to write the unbalanced equation and determine the coefficients for the most complex molecule or compound.
What order should atoms be balanced when setting a chemical reaction equation?
-When balancing a chemical reaction, atoms should be balanced in the following order: cations (positively charged ions), anions (negatively charged ions), hydrogen atoms, and oxygen atoms.
What is the significance of using fractional coefficients in balancing equations?
-Fractional coefficients may be used when balancing equations to maintain the simplest ratios of atoms. These fractions are then multiplied by a common factor to convert them into whole numbers.
How do you handle fractional coefficients when simplifying a chemical reaction equation?
-To simplify a chemical reaction equation with fractional coefficients, multiply all coefficients by the denominator of the fraction to convert them into whole numbers.
Can you provide an example of how to balance a chemical reaction equation?
-For example, in the reaction between aluminum and hydrochloric acid (Al + HCl → AlCl3 + H2), the aluminum coefficient is set to 1, the chlorine coefficient is adjusted to balance the anions, and then the hydrogen atoms are balanced by multiplying the hydrogen gas coefficient by 3/2, eventually simplifying the coefficients to whole numbers.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)