Carbon Element 💎 - Periodic Table | Properties, Uses & More!
Summary
TLDRIn this video, we explore the fascinating world of carbon, a non-metal element with atomic number 6. From ancient uses of charcoal to its discovery as an element in 1789, carbon plays a crucial role in the universe and life on Earth. We delve into carbon's diverse allotropes, including graphite, diamond, and graphene, and highlight its importance in fields like healthcare, textiles, and industry. Carbon’s versatility in bonding makes it the 'king of the elements,' vital in life forms and fossil fuels. We also discuss the carbon cycle and ways to reduce our carbon footprint. Plus, we uncover the history of carbon in tattoos and car tires!
Takeaways
- 😀 Carbon is a non-metal element with atomic number 6 and is the fourth most common element in the universe.
- 😀 The name 'carbon' comes from the Latin word 'carbo', meaning coal or charcoal.
- 😀 Carbon has been known since ancient times, with evidence of its use as charcoal dating back to 3,750 BC.
- 😀 French chemist Antoine Lavoisier first identified carbon as an element in 1789.
- 😀 Carbon is created within stars and supernovae through the triple-alpha process, not in the Big Bang.
- 😀 Carbon is tetravalent, meaning it can form stable bonds with itself, allowing it to exist in various allotropes.
- 😀 Common allotropes of carbon include graphite, diamond, and amorphous carbon.
- 😀 Graphene, a single layer of carbon atoms, is the thinnest and strongest material known, discovered in 2004.
- 😀 Carbon is a crucial element for life, found in DNA, proteins, and making up about 19% of the human body by weight.
- 😀 The carbon cycle continuously transforms carbon from one form to another, allowing it to be reused and recycled.
- 😀 The carbon footprint refers to the total amount of greenhouse gases generated by human actions, and small efforts like planting trees and recycling can help reduce it.
Q & A
What is carbon and where is it located in the periodic table?
-Carbon is a non-metal element with the atomic number 6. It belongs to group 14 in the periodic table.
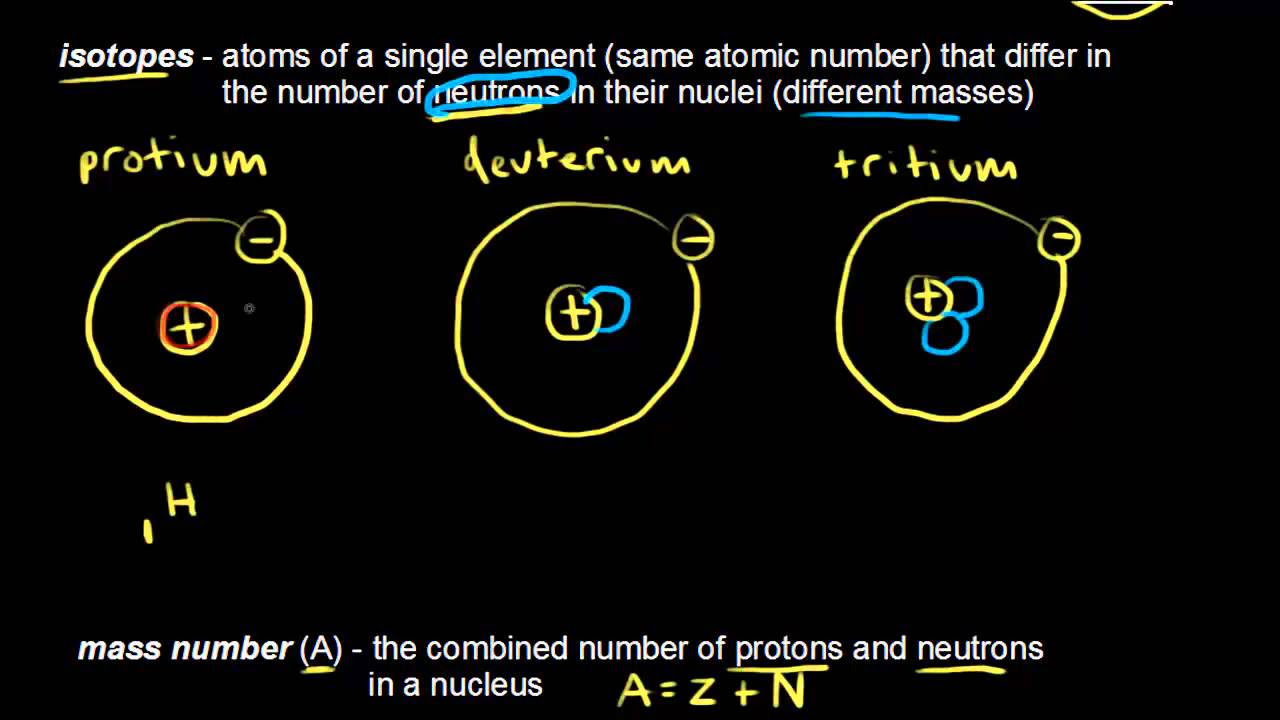
How many protons, electrons, and neutrons does a typical carbon atom have?
-A typical carbon atom consists of six protons, six electrons, and six neutrons.
What does the name 'carbon' come from?
-The name 'carbon' comes from the Latin word 'carbo,' which means coal, charcoal, or ember.
How long has carbon been known, and how was it used in ancient times?
-Carbon has been known since ancient times, particularly in the form of charcoal, as early as 3,750 BC. The ancient Egyptians and Sumerians used charcoal to reduce various metals.
When was carbon identified as an element?
-Carbon was identified as an element for the first time in 1789 by French chemist Antoine Lavoisier.
What are the most common forms of carbon?
-The most common allotropes of carbon are graphite, diamond, and amorphous carbon.
What is the difference between graphite and diamond in terms of their structure and properties?
-Graphite consists of loosely connected sheets of carbon atoms, making it dull black, electrically conductive, and soft. Diamond, on the other hand, has a three-dimensional structure with strong bonds, making it transparent, non-conductive, and the hardest substance on Earth.
What is amorphous carbon, and where can it be found?
-Amorphous carbon lacks a defined crystal structure. Examples include charcoal, soot, and coke, which are used in industries like textiles and healthcare.
What are fullerenes and graphene, and what are their key discoveries?
-Fullerenes are carbon allotropes where atoms are arranged in closed shells, discovered in 1985. Graphene, a single layer of carbon atoms arranged in hexagons, was discovered in 2004 and is known for being the thinnest, strongest material ever known.
What makes carbon unique in the chemistry world?
-Carbon is called the 'king of the elements' due to its ability to form stable covalent bonds with itself and other elements. It is capable of forming nearly 10 million compounds, more than any other element.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)