F197 - Proses proses termodinamika ( isobaris,isokoris,isotermis,adiabatis) : Teori + Contoh soal
Summary
TLDRThis script explains key thermodynamic processes such as isobaric, isochoric, isothermal, and adiabatic processes. It details how each process affects variables like pressure, volume, temperature, and energy. The discussion includes essential thermodynamic formulas for calculating work, heat change, and internal energy. Additionally, it provides example problems to illustrate the practical application of these concepts, including the calculation of work done and energy changes in different thermodynamic processes. The video aims to make complex thermodynamic principles more accessible for students and enthusiasts.
Takeaways
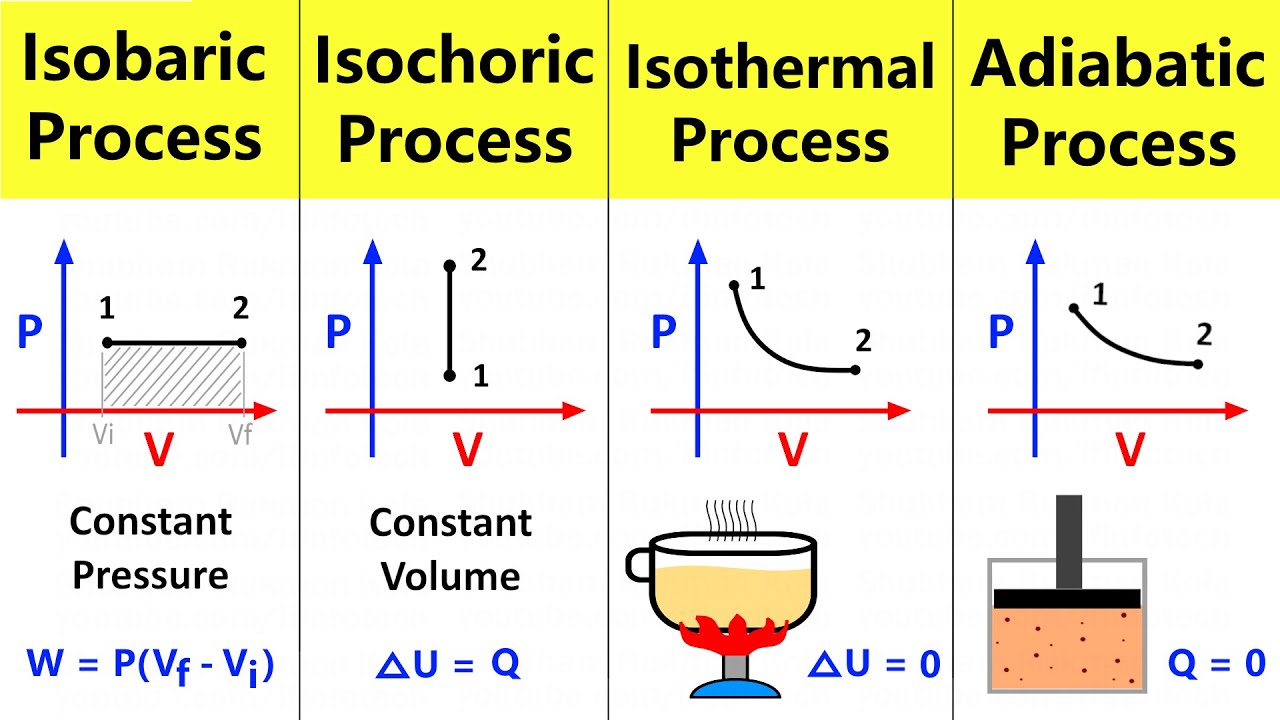
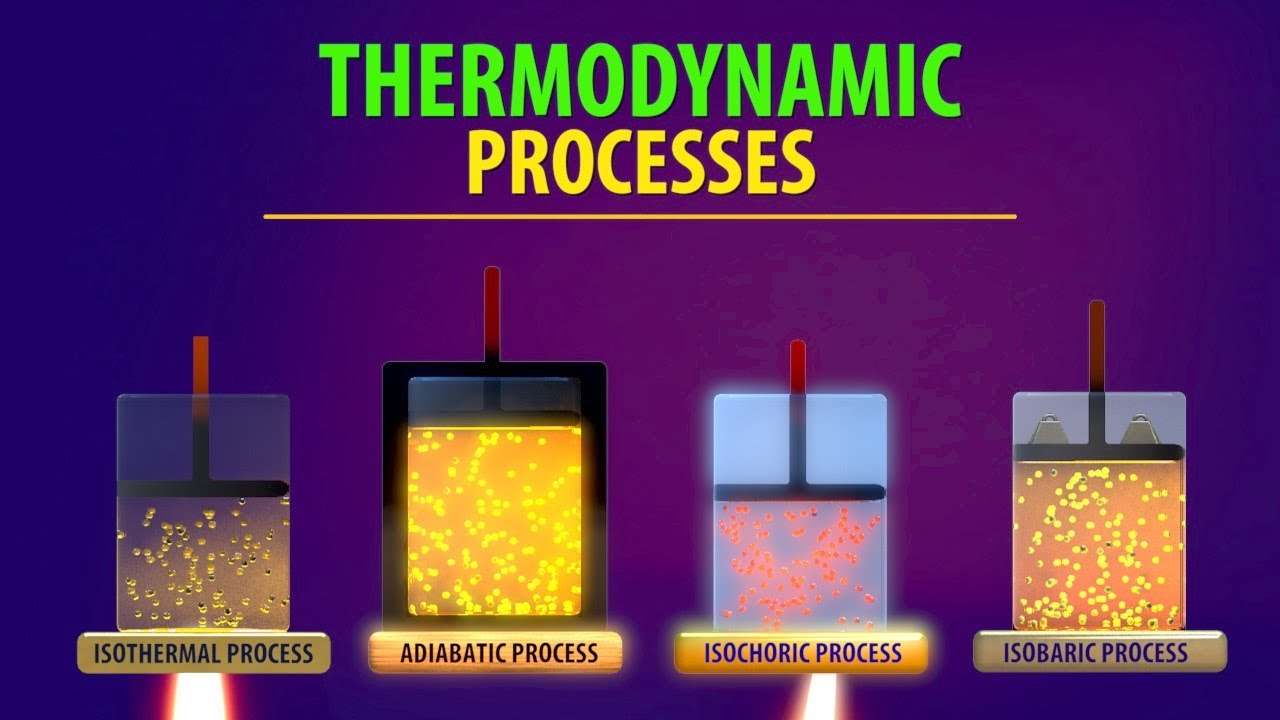
- 😀 The script explains four types of thermodynamic processes: isobaric, isochoric, isothermal, and adiabatic.
- 😀 In isobaric processes, the pressure remains constant, and the volume changes, which can lead to either expansion or compression of the gas.
- 😀 In isochoric processes, the volume remains constant, and there is no work done as the volume doesn’t change.
- 😀 Isothermal processes occur at constant temperature, with the relationship between pressure and volume governed by Boyle’s Law (P1V1 = P2V2).
- 😀 In adiabatic processes, there is no heat exchange between the system and its surroundings, and the relationship between pressure and volume is governed by PV^γ = constant.
- 😀 The change in heat (ΔQ) in a gas system can be calculated using the formula ΔQ = C * ΔT, where C is the specific heat capacity and ΔT is the temperature change.
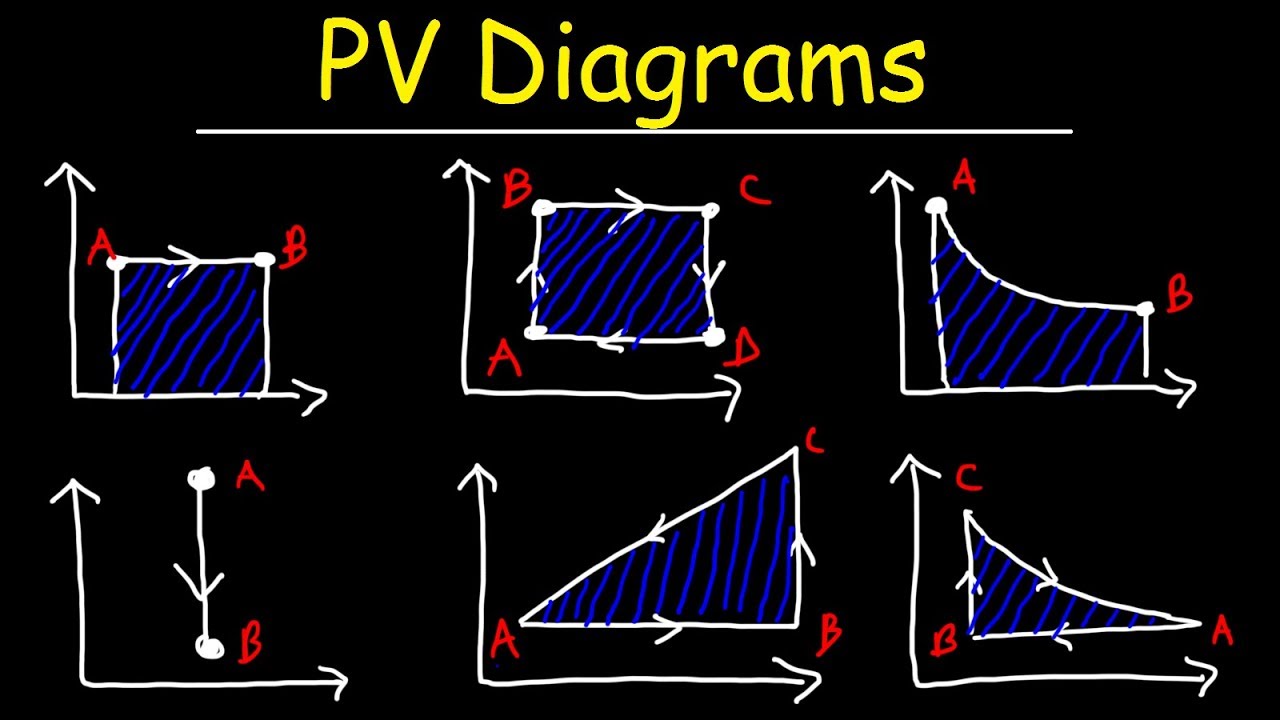
- 😀 The work done (W) in any thermodynamic process can be calculated as P * ΔV, where P is pressure and ΔV is the change in volume.
- 😀 The script emphasizes the first law of thermodynamics, stating that the change in internal energy equals the heat added to the system minus the work done by the system.
- 😀 For isothermal processes, the work done is calculated using the formula W = nRT * ln(V2/V1), where n is the number of moles, R is the gas constant, and V1 and V2 are initial and final volumes.
- 😀 The script provides a practical example of calculating work in a cycle, involving processes like isobaric and isochoric, to determine total work done in the system.
- 😀 The calculation of changes in internal energy and work in thermodynamic processes can be approached through step-by-step formulas, making the understanding of each process clearer.
Q & A
What is an isobaric process in thermodynamics?
-An isobaric process is one in which the pressure remains constant throughout the process. During this process, the volume of the gas changes, but the pressure stays the same. The equation for work done in an isobaric process is W = P * ΔV.
How do you calculate heat change (Q) in an isobaric process?
-In an isobaric process, the heat change (Q) can be calculated using the formula Q = C_P * ΔT, where C_P is the specific heat capacity at constant pressure and ΔT is the temperature change of the gas.
What happens to volume and pressure in an isochoric process?
-In an isochoric process, the volume of the gas remains constant, which means there is no change in volume (ΔV = 0). As a result, no work is done in the process. The heat change is related to the change in internal energy.
How do you calculate work (W) in an isochoric process?
-Since there is no volume change in an isochoric process (ΔV = 0), no work is done (W = 0) during the process.
What is an isothermal process?
-An isothermal process is one where the temperature of the gas remains constant throughout the process. This implies that the internal energy change is zero (ΔU = 0). The work done in an isothermal process can be calculated using the formula W = nRT * ln(V2/V1), where V2 and V1 are the final and initial volumes, respectively.
What is the key characteristic of an adiabatic process?
-In an adiabatic process, there is no heat exchange between the system and its surroundings. The system does not absorb or release heat, and the energy change is entirely due to work done on or by the gas. The relationship between pressure and volume in an adiabatic process is given by PV^γ = constant.
How do you calculate work done in an adiabatic process?
-The work done in an adiabatic process can be calculated using the formula W = (P2V2 - P1V1) / (γ - 1), where γ is the adiabatic index (ratio of specific heats), and P1, V1, P2, and V2 are the initial and final pressure and volume values.
What does the first law of thermodynamics state for any thermodynamic process?
-The first law of thermodynamics states that the change in internal energy of the system (ΔU) is equal to the heat added to the system (Q) minus the work done by the system (W): ΔU = Q - W. This applies to all thermodynamic processes, including isobaric, isochoric, isothermal, and adiabatic processes.
In the context of the isothermal process, what happens to the internal energy of an ideal gas?
-In an isothermal process for an ideal gas, since the temperature remains constant, the internal energy (which is a function of temperature) does not change. Therefore, ΔU = 0 in an isothermal process.
How does the work in an isobaric process differ from that in an isothermal process?
-In an isobaric process, the work done by the gas is calculated as W = P * ΔV, where the pressure is constant and the volume changes. In an isothermal process, the work is calculated using W = nRT * ln(V2/V1), where the temperature remains constant, and the relationship between pressure and volume is governed by Boyle's law.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)