Temperature: Crash Course Physics #20
Summary
TLDRThis episode of Crash Course Physics explores the concept of temperature and its effects on materials, specifically focusing on thermal expansion. The video explains how bridges use expansion joints to accommodate changes in temperature, preventing stress and damage. It also delves into the behavior of gases using the ideal gas law, which relates pressure, volume, temperature, and the amount of gas. The video demonstrates how these concepts play out in everyday situations, like the air pressure changes in a car due to temperature fluctuations. The episode is both informative and practical, offering insights into thermodynamics and gas laws.
Takeaways
- 😀 Expansion joints are necessary in bridges to account for thermal expansion and contraction, preventing potential structural failure.
- 😀 Temperature is a measure of kinetic energy, which reflects how fast the atoms and molecules in a substance are moving.
- 😀 Heat transfer occurs from hotter systems to colder ones, and thermal equilibrium happens when there is no heat transfer.
- 😀 Thermal expansion causes solids to expand when heated and contract when cooled, with the amount of change depending on the material and temperature change.
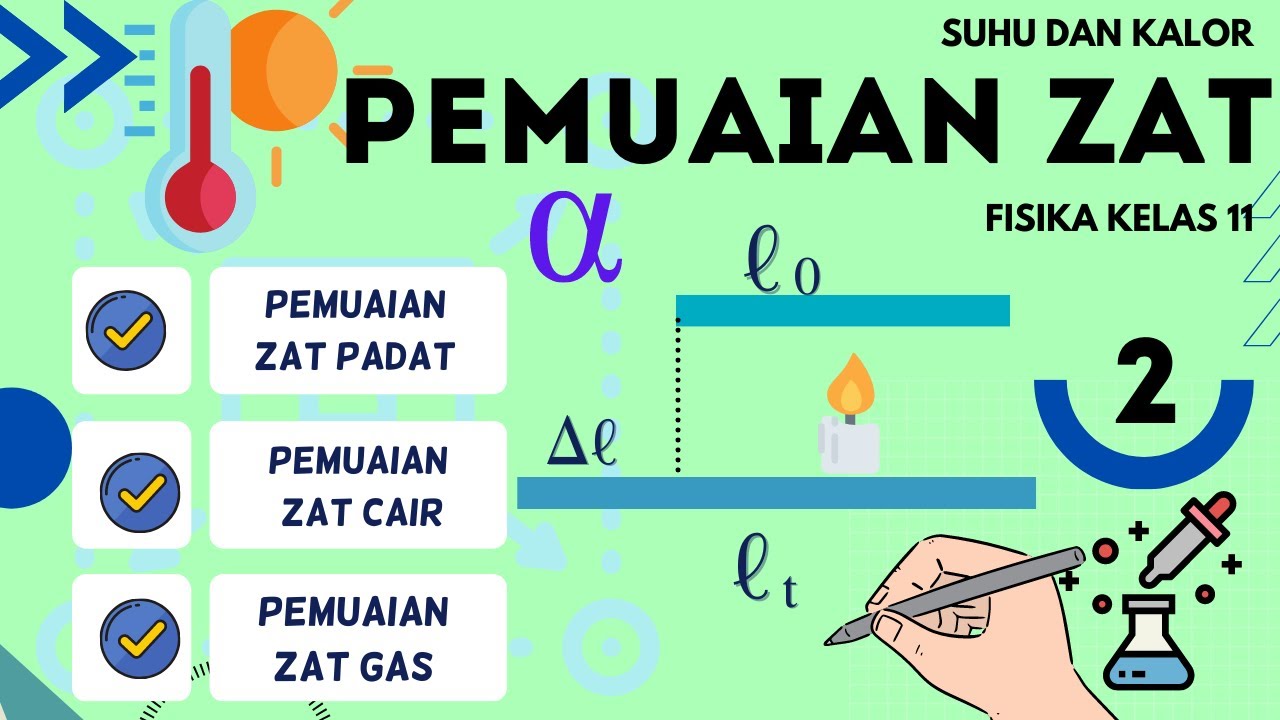
- 😀 The equation for linear expansion describes how the length of a material changes with temperature, depending on the material’s coefficient of linear expansion.
- 😀 Without expansion joints, materials like concrete would experience stress and could break due to temperature-induced changes in length.
- 😀 Volume expansion occurs when all three dimensions of a material change, and is influenced by the material’s coefficient of volume expansion.
- 😀 Gases also experience temperature-driven changes, affecting their pressure, volume, and the number of gas molecules, which is governed by the ideal gas law.
- 😀 The three fundamental laws of ideal gases—Boyle’s Law, Charles’s Law, and Gay-Lussac’s Law—explain how pressure, temperature, and volume interact under various conditions.
- 😀 The ideal gas law (PV = nRT) combines Boyle’s, Charles’s, and Gay-Lussac’s laws into one equation to describe the relationship between pressure, volume, temperature, and the number of gas molecules.
Q & A
What are expansion joints in bridges and why are they necessary?
-Expansion joints in bridges are cracks that contain metal grating, designed to allow the concrete to expand and contract with temperature changes. Without these joints, the concrete would experience too much stress and could fail.
What is temperature a measure of, and how does it relate to the motion of atoms and molecules?
-Temperature is a measure of the kinetic energy in a system, which is the energy of motion. The hotter something is, the more kinetic energy its atoms and molecules have, meaning they move more rapidly.
What is thermal equilibrium?
-Thermal equilibrium is when two systems have no heat transfer between them because they are at the same temperature.
How does temperature affect the size of materials?
-Temperature changes can cause materials to expand or contract. Typically, an increase in temperature will make a solid expand, and a decrease will cause it to contract.
What is linear expansion, and how is it calculated?
-Linear expansion refers to the change in length of an object due to temperature change. It is calculated using the formula: ΔL = α * L₀ * ΔT, where α is the coefficient of linear expansion, L₀ is the initial length, and ΔT is the change in temperature.
How does the coefficient of linear expansion affect the thermal expansion of a material?
-The coefficient of linear expansion (α) represents how much a material will expand or contract for each degree change in temperature. Materials with a higher coefficient of expansion will experience more significant changes in length with temperature changes.
What is volume expansion, and how does it differ from linear expansion?
-Volume expansion is the change in the volume of an object due to temperature changes, whereas linear expansion affects only the length. Volume expansion occurs in all three dimensions, and is calculated using the formula: ΔV = β * V₀ * ΔT, where β is the coefficient of volume expansion, V₀ is the initial volume, and ΔT is the temperature change.
How do gases respond to temperature changes, and what laws govern these changes?
-Gases expand when heated and contract when cooled, and these changes are governed by laws such as Boyle's Law (pressure and volume are inversely related at constant temperature), Charles’s Law (volume and temperature are directly related at constant pressure), and Gay-Lussac’s Law (pressure and temperature are directly related at constant volume).
What is the Ideal Gas Law, and how does it relate pressure, volume, temperature, and the number of moles?
-The Ideal Gas Law is a mathematical relationship that connects pressure, volume, temperature, and the number of moles of a gas. It is expressed as PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature in Kelvin.
How does the temperature inside a car affect the number of moles of air inside?
-As the temperature inside a car increases, the volume of air remains constant, and the pressure outside is the same, causing a decrease in the number of moles of air inside. This is an application of the Ideal Gas Law, where a higher temperature results in fewer moles of air.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)