Unsur dan Tabel Periodik Unsur
Summary
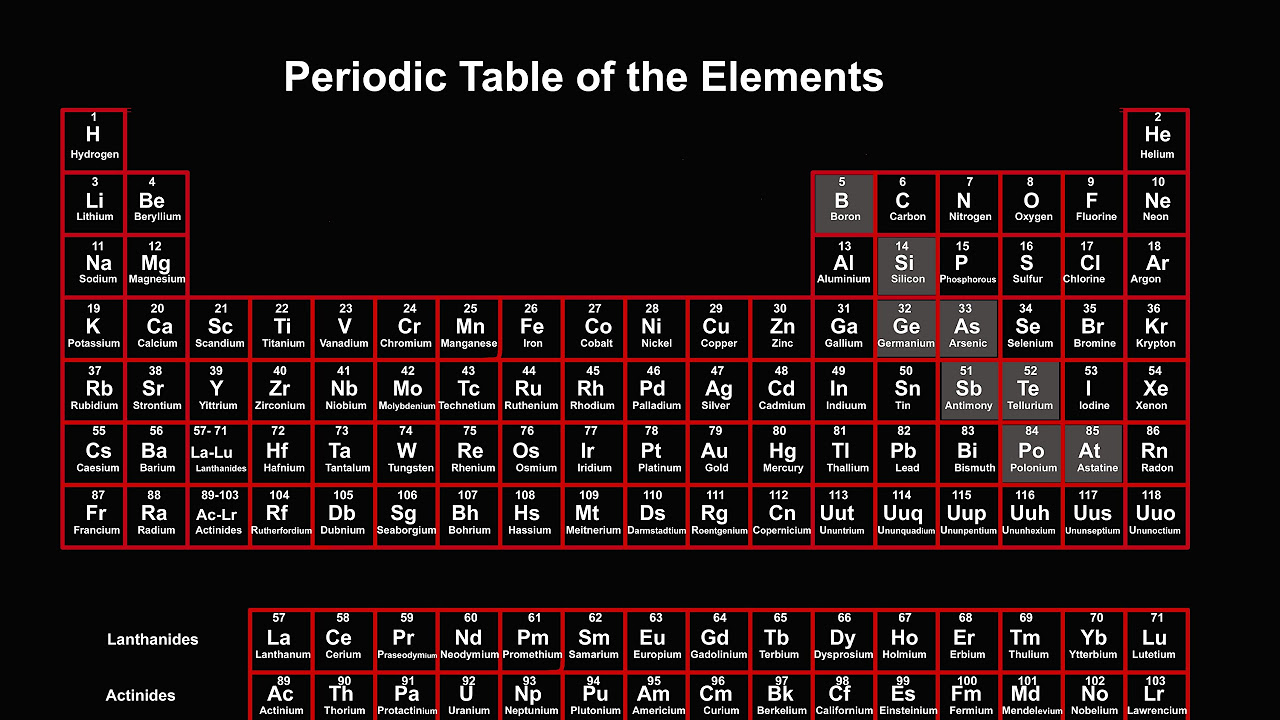
TLDRThe video discusses the composition of matter, highlighting elements that make up various substances, such as metals and gases. It explains that elements are pure chemical substances that cannot be broken down further, with a total of 118 known elements categorized in the periodic table. The table features 7 periods and 18 groups, with specific groups such as alkali metals and noble gases receiving special names. The presentation also covers the characteristics of metals, nonmetals, and metalloids, and invites viewers to subscribe for more educational content.
Takeaways
- 😀 Elements are the fundamental substances that make up all matter around us.
- 😀 There are 118 known elements, divided into natural and synthetic types.
- 😀 Elements are represented by symbols, often derived from their names.
- 😀 The periodic table organizes elements into periods (rows) and groups (columns).
- 😀 The International Union of Pure and Applied Chemistry (IUPAC) recommended a numbering system for groups from 1 to 18.
- 😀 The periodic table contains 32 groups, but is typically displayed in a condensed format.
- 😀 Lanthanides and actinides are special categories of elements, highlighted in the table.
- 😀 Elements are categorized into metals, nonmetals, and metalloids based on their properties.
- 😀 Metals, except mercury, are solid at room temperature and conduct heat and electricity well.
- 😀 Nonmetals can exist as gases, liquids, or solids, with distinct properties compared to metals.
Q & A
What are chemical elements?
-Chemical elements are substances that cannot be broken down into simpler substances and are the building blocks of all matter.
How many chemical elements are currently known?
-As of now, there are 118 known chemical elements.
What is the periodic table?
-The periodic table is a tabular arrangement of elements, organized by their atomic number, electron configuration, and recurring chemical properties.
How is the periodic table structured?
-The periodic table consists of seven rows called periods and 18 columns known as groups or families.
What distinguishes natural elements from synthetic ones?
-Natural elements occur in nature, while synthetic elements are artificially created in laboratories.
What are the classifications of elements in the periodic table?
-Elements are classified into metals, nonmetals, and metalloids, with metals generally being solid at room temperature except for mercury.
What are the specific groups mentioned in the transcript?
-The transcript mentions groups such as alkali metals, alkaline earth metals, halogens, noble gases, and transition metals.
What color coding is used in the periodic table for different groups?
-Different groups in the periodic table are color-coded: alkali metals (yellow), alkaline earth metals (blue), halogens (green), noble gases (purple), and transition metals (green).
What does the term 'transition metals' refer to?
-Transition metals are elements that have partially filled d orbitals and are located in groups 3-12 of the periodic table.
What is the significance of the symbols assigned to elements?
-Each element is assigned a unique chemical symbol, usually derived from its name, which is used for identification in chemical equations and formulas.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)