Manufacturing Molybdenum-99 for medicine at TUM's Research Neutron Source
Summary
TLDRThe video highlights the critical role of technetium-99m in modern medicine, particularly for diagnosing diseases, and addresses the looming production shortages due to decreasing nuclear reactor availability. A new facility at the Research Neutron Source FRM II in Munich aims to mitigate these shortages by producing technetium-99m from irradiated uranium targets. Engineers are overcoming technical challenges in the facility's design and ensuring safety through rigorous testing and regulatory compliance. The facility's operation is scheduled for test irradiations in 2018, with routine production anticipated by 2019, promising to enhance the supply of this vital medical isotope.
Takeaways
- 😀 Technetium-99m is crucial for modern medicine, used for imaging diseases in organs like the heart and thyroid.
- 📊 It is utilized around 3 million times annually in Germany and approximately 30 million times worldwide.
- ⚠️ The availability of nuclear reactors for technetium-99m production is declining, increasing the risk of shortages.
- 🏗️ A new isotope production plant is being established at the Research Neutron Source FRM II in Munich.
- 🔧 Dr. Heiko Gerstenberg oversees the irradiation facilities, aiming to address the technetium-99m supply shortage.
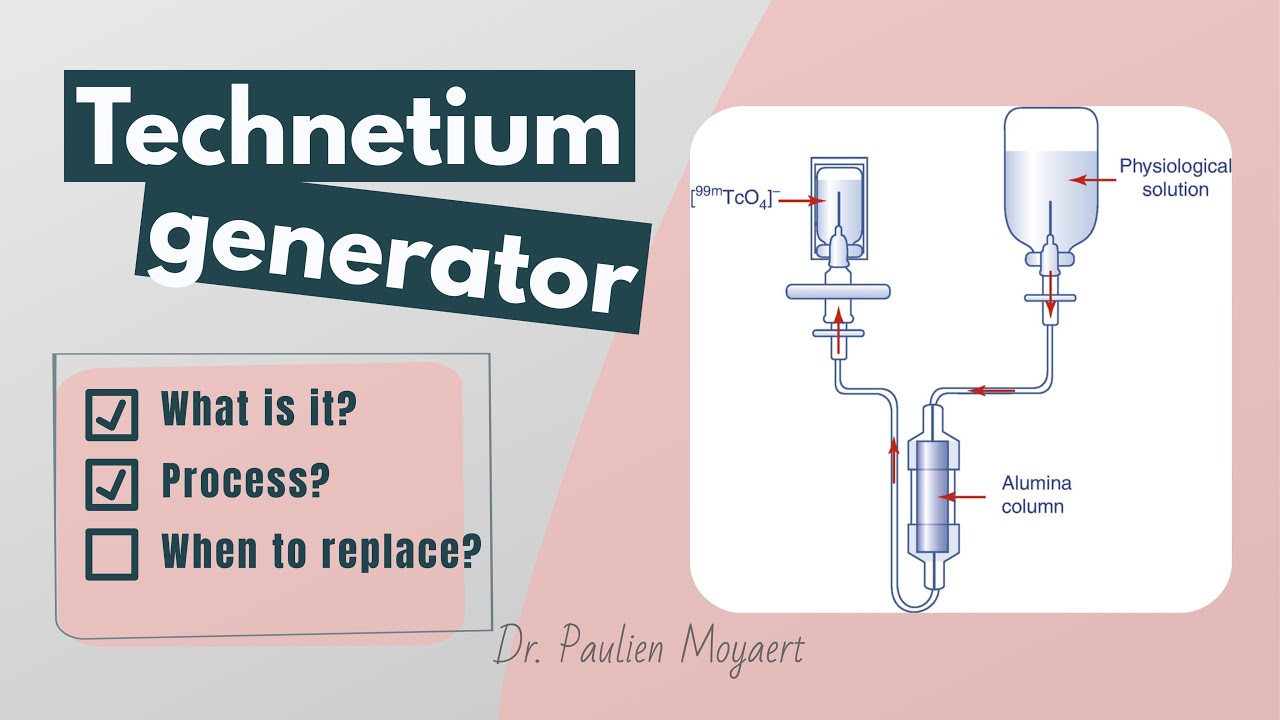
- 🧪 The facility uses uranium targets irradiated with neutrons to produce molybdenum-99 (Mo-99), the precursor to technetium-99m.
- ⏳ Mo-99 decays to technetium-99m with a half-life of only 66 hours, necessitating rapid delivery to medical facilities.
- 🔍 Engineers are constructing a 1:1 scale model of the irradiation system to ensure functionality and safety.
- 📄 A nuclear licensing procedure is underway, requiring extensive documentation and evaluation by regulatory bodies.
- 🚀 Routine production of technetium-99m is anticipated to begin in 2019, following successful testing and installation.
Q & A
What is technetium-99m and why is it important in modern medicine?
-Technetium-99m is a radionuclide used in medical imaging to visualize diseases in organs such as the heart, thyroid, and bones. Its importance lies in its ability to provide detailed diagnostic information while minimizing patient radiation exposure.
How often is technetium-99m used globally?
-Technetium-99m is used approximately 30 million times each year worldwide, with around 3 million uses in Germany alone.
What challenges are currently facing the production of technetium-99m?
-The production of technetium-99m is threatened by a decreasing number of operational nuclear reactors, leading to concerns about potential shortages.
Where is the new facility for producing technetium-99m being established?
-The new facility is being established at the Research Neutron Source FRM II, located at the Technical University of Munich in Garching, Germany.
Who is responsible for the irradiation facilities at the FRM II?
-Dr. Heiko Gerstenberg is responsible for overseeing the irradiation facilities at the Research Neutron Source FRM II.
What is the process used to produce molybdenum-99 at the facility?
-Uranium targets are irradiated with neutrons for six days to produce molybdenum-99, which then decays into technetium-99m.
What are the characteristics of the targets used in the irradiation process?
-The targets are slim fuel plates covered by an aluminum alloy and hold 4 grams of uranium, which is necessary for the production of technetium-99m.
How does the facility ensure safety and functionality during testing?
-Testing is conducted underwater to identify potential leaks and ensure that the facility operates properly, allowing for immediate detection of issues like leaking through bubble formation.
What is the expected timeline for the facility's operation?
-Test irradiations are scheduled to begin in 2018, with routine production of technetium-99m expected to start in 2019 at the earliest.
What role does regulatory approval play in the operation of the new facility?
-The facility is undergoing a nuclear licensing procedure, where documents are submitted to regulatory experts to obtain approval for installation and operation, ensuring compliance with safety standards.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)