How Small Is An Atom? Spoiler: Very Small.
Summary
TLDRThis video script explores the fascinating world of atoms, illustrating their unimaginable smallness and complexity. It compares atom sizes to everyday objects, explaining the structure of atoms made of neutrons, protons, and electrons. The script dives into quantum mechanics, introducing concepts like quarks, orbitals, and quantum fluctuations. It emphasizes the mysterious and often counterintuitive nature of atomic particles and how they form the building blocks of the universe. The message concludes by encouraging continued support for scientific research as we uncover more about this enigmatic realm.
Takeaways
- 🔬 Atoms are incredibly small; 500,000 carbon atoms stacked on top of each other are about as thick as a human hair.
- 👊 Your fist contains trillions of atoms, and if one atom were the size of a marble, your fist would be as big as Earth.
- 📏 Atoms are mostly empty space, but this 'space' is filled with quantum fluctuations that affect how particles interact.
- 🔗 Atoms consist of three elementary particles: protons, neutrons, and electrons.
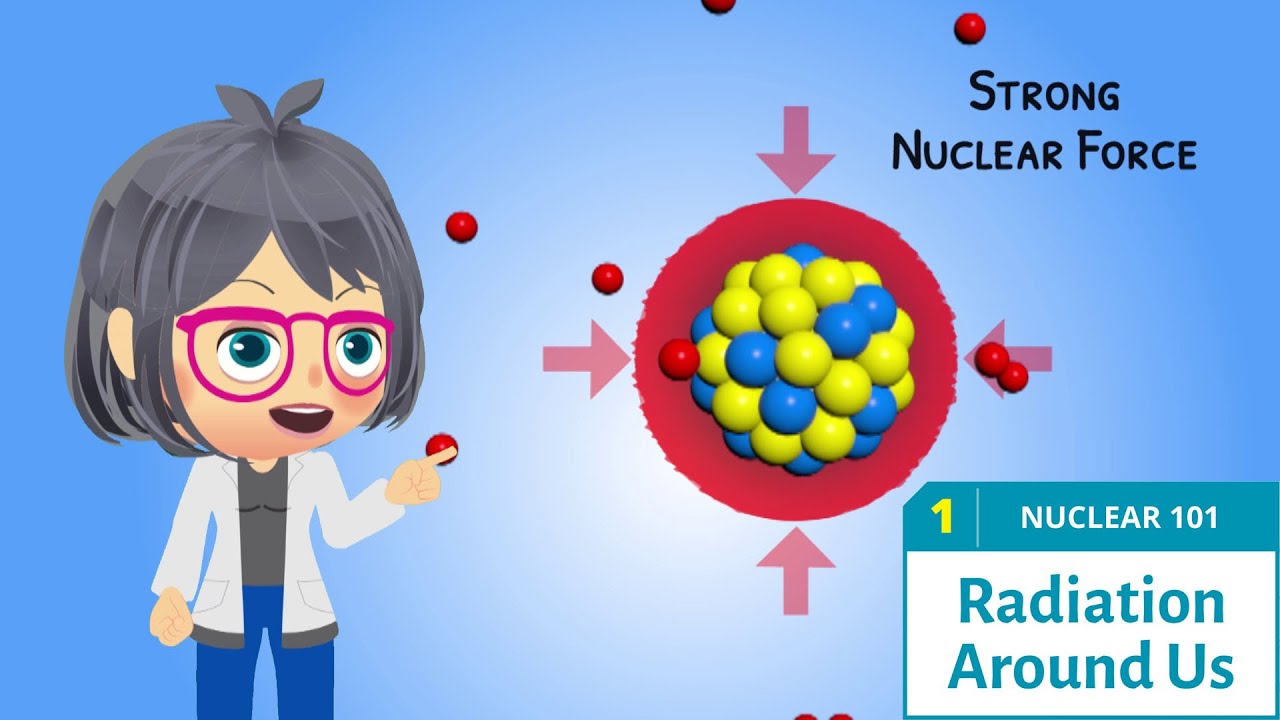
- 💥 Protons and neutrons form the atom's core, held together by the strong interaction, one of the four fundamental forces in the universe.
- 🌀 Electrons orbit the nucleus at incredible speeds (2,200 km/s), and they are considered fundamental particles like quarks.
- 📉 99.999999999999% of an atom’s volume is empty space, but that space is filled with energy fields and quantum fluctuations.
- 🌌 The core and electrons of atoms take up very little space. For example, if you removed all the empty space from the atoms in the Empire State Building, it would shrink to the size of a rice grain.
- 🌟 In neutron stars, atom cores are so densely packed that three Suns’ worth of mass can fit into a space only a few kilometers wide.
- 🧪 All atoms of the same element are identical, and every hydrogen atom in the universe is the same as the hydrogen in the Sun.
Q & A
How small are atoms compared to everyday objects?
-Atoms are incredibly small. For example, about 500,000 carbon atoms stacked on top of each other would be about as thick as a single human hair.
If one atom in a fist were as big as a marble, how big would the fist be?
-If one atom were the size of a marble, your fist would be about the size of Earth.
What happens when we zoom in on the cells in the tip of your finger?
-If the tip of your little finger was the size of a room, one cell would also fill the room, and within that cell, proteins and atoms would be even smaller, represented by grains of sand.
What are atoms made of?
-Atoms consist of three elementary particles: neutrons, protons, and electrons. Neutrons and protons form the atom’s core, which is held together by the strong interaction.
What are quarks, and how small are they?
-Quarks are the fundamental particles that make up protons and neutrons. We believe quarks might be point-like and zero-dimensional, but their exact size is unknown.
How fast do electrons move around the atom's core?
-Electrons orbit the atom’s core at about 2,200 km/s, which is fast enough to travel around the Earth in just over 18 seconds.
Is most of an atom just empty space?
-While 99.999999999999% of an atom appears to be empty space, it is actually filled with quantum fluctuations and fields that influence how charged particles interact.
If we removed the empty space between atoms, how big would the Empire State Building be?
-If you removed all the empty space between the atom cores in the Empire State Building, it would be reduced to about the size of a grain of rice.
How do electrons exist around the atom's core?
-Electrons behave both as particles and as waves. They exist in probability clouds called orbitals, where they are likely to be found with 95% certainty, but there is a small chance they could be found much farther away.
What do we need to create different elements in the universe?
-By adding different numbers of protons, neutrons, and electrons, we get different elements. For example, hydrogen consists of one proton and one electron, while helium adds a neutron.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)