04_02 Solubility Rules
Summary
TLDRThis educational script introduces solubility rules for ionic compounds, crucial for understanding precipitation reactions. It explains that alkali metals and ammonium ions are always soluble, while certain anions like sulfates, chlorides, and bromides are soluble with exceptions. The script emphasizes memorizing these rules and exceptions to predict if a compound will dissolve in water, using examples to demonstrate how to apply these rules.
Takeaways
- 🧪 Chemical equations represent chemical reactions, and understanding them is fundamental to studying different types of chemical reactions.
- 💧 Solubility in water is a key concept for predicting precipitation reactions, which involve the formation of an insoluble product.
- 📜 There are solubility rules to determine if an ionic compound is soluble or insoluble in water, and these rules can change over time.
- 🔍 Alkali metals and the ammonium ion are cations that are always soluble in water, regardless of the anion they are paired with.
- 📌 Four anions (not including fluoride) are always soluble when paired with any cation.
- 📝 There are exceptions to the solubility of halides, sulfates, and hydroxides that need to be memorized.
- 🚫 Insoluble compounds are generally insoluble, but exceptions exist where certain anions paired with specific cations can be soluble.
- 📚 The solubility rules are not easy to memorize and may require frequent revision.
- 🔎 When determining solubility, start with the rules and then check for exceptions to those rules.
- 📉 Even compounds deemed insoluble can dissolve in very small amounts, such as parts per million, billion, or trillion.
- 🌐 The concept of memorize list is a reliable source for the current solubility rules, and it should be consulted regularly for accuracy.
Q & A
What is the purpose of balancing chemical equations?
-Balancing chemical equations represents chemical reactions that occur, ensuring that the number of atoms for each element is equal on both sides of the equation.
What drives precipitation reactions?
-Precipitation reactions are driven by the solubility or insolubility of ionic compounds in water.
How do you determine if an ionic compound is soluble or insoluble in water?
-You determine solubility by using solubility rules, which outline which compounds dissolve in water and which do not.
What is meant by the term 'soluble' in relation to ionic compounds?
-'Soluble' means that an ionic compound dissolves in water, while 'insoluble' means that it does not dissolve.
Why is it important to memorize solubility rules?
-Memorizing solubility rules helps predict whether a compound will dissolve in water, which is key to understanding and predicting the outcome of precipitation reactions.
Which cations are always soluble with no exceptions?
-Alkali metals (e.g., sodium, potassium) and the ammonium ion (NH4+) are always soluble with no exceptions.
What are some anions that are always soluble with no exceptions?
-Anions such as nitrate (NO3-), acetate (C2H3O2-), chlorate (ClO3-), and perchlorate (ClO4-) are always soluble.
What are the exceptions to the solubility of halides (chloride, bromide, iodide)?
-Halides are generally soluble, except when they are combined with silver (Ag+), lead (Pb2+), or mercury (Hg2 2+), making them insoluble.
Why are some sulfate compounds insoluble?
-While sulfate ions are generally soluble, exceptions occur with cations like barium (Ba2+), calcium (Ca2+), lead (Pb2+), and strontium (Sr2+), which form insoluble compounds with sulfate.
How can you determine if a compound is insoluble based on the solubility rules?
-If a compound contains anions like hydroxide (OH-) or sulfide (S2-) without exceptions, it is generally insoluble unless paired with cations like alkali metals or ammonium, which make them soluble.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

13.1 Compounds in Aqueous Solutions
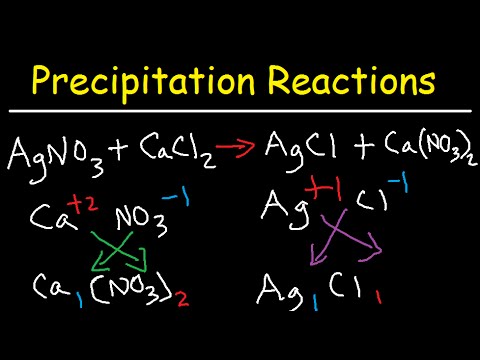
Precipitation Reactions and Net Ionic Equations - Chemistry
Precipitation Reactions: Crash Course Chemistry #9
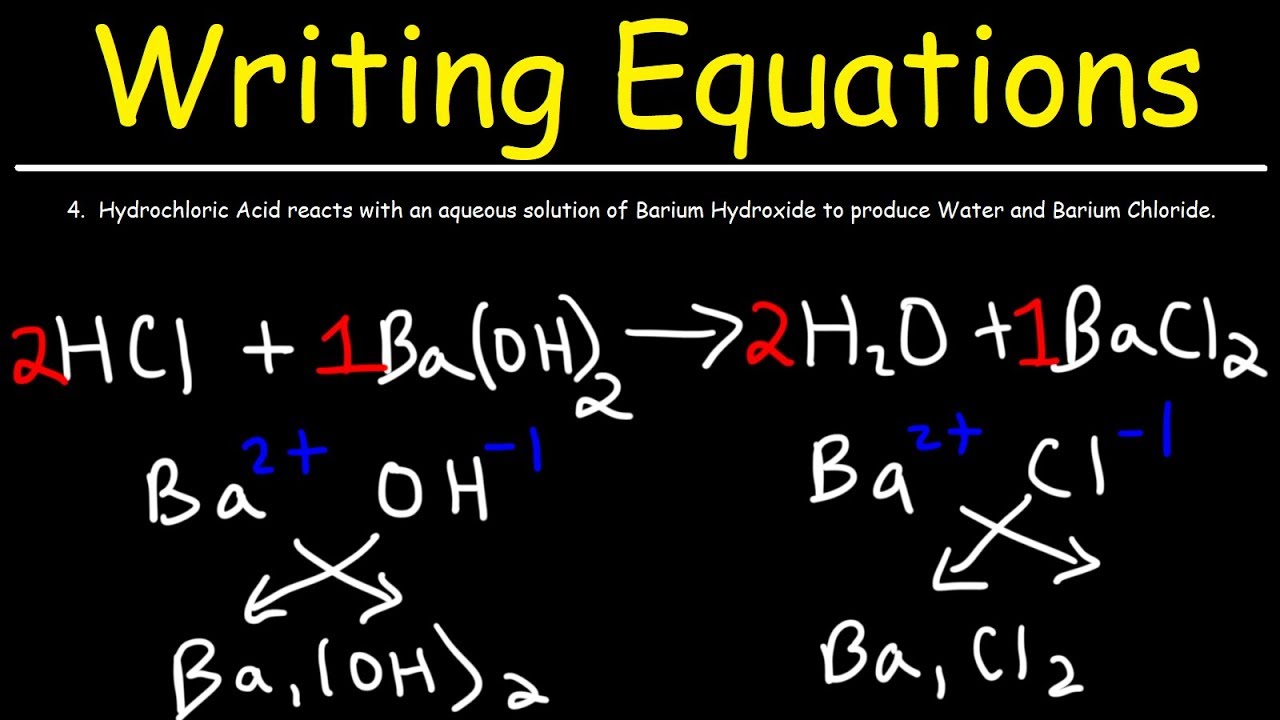
How To Write Chemical Equations From Word Descriptions
Solubility Product Constant (Ksp)

Cálculo do NOX | Número de Oxidação | Regras Práticas | Eletroquímica | Aula 02
5.0 / 5 (0 votes)