Naming Esters - Organic Chemistry IUPAC Naming by Leah4sci
Summary
TLDRIn this educational video, Laya from Live for Sicom explains the process of naming esters in organic chemistry. She clarifies the structure of esters, differentiating them from carboxylic acids, aldehydes, and ethers. The video demonstrates how to derive ester names from carboxylic acids and alcohols, emphasizing the importance of the carbonyl group's position. Examples are provided to illustrate the naming process, including handling of substituents and multiple functional groups. The video is part of a series aiding students in organic chemistry, with resources for further learning and tutoring mentioned.
Takeaways
- 🧪 Ester molecules have an R group attached to a carbonyl group (C=O) bonded to an oxygen.
- 🔍 Ester groups can be distinguished from carboxylic acids and aldehydes by their molecular structure.
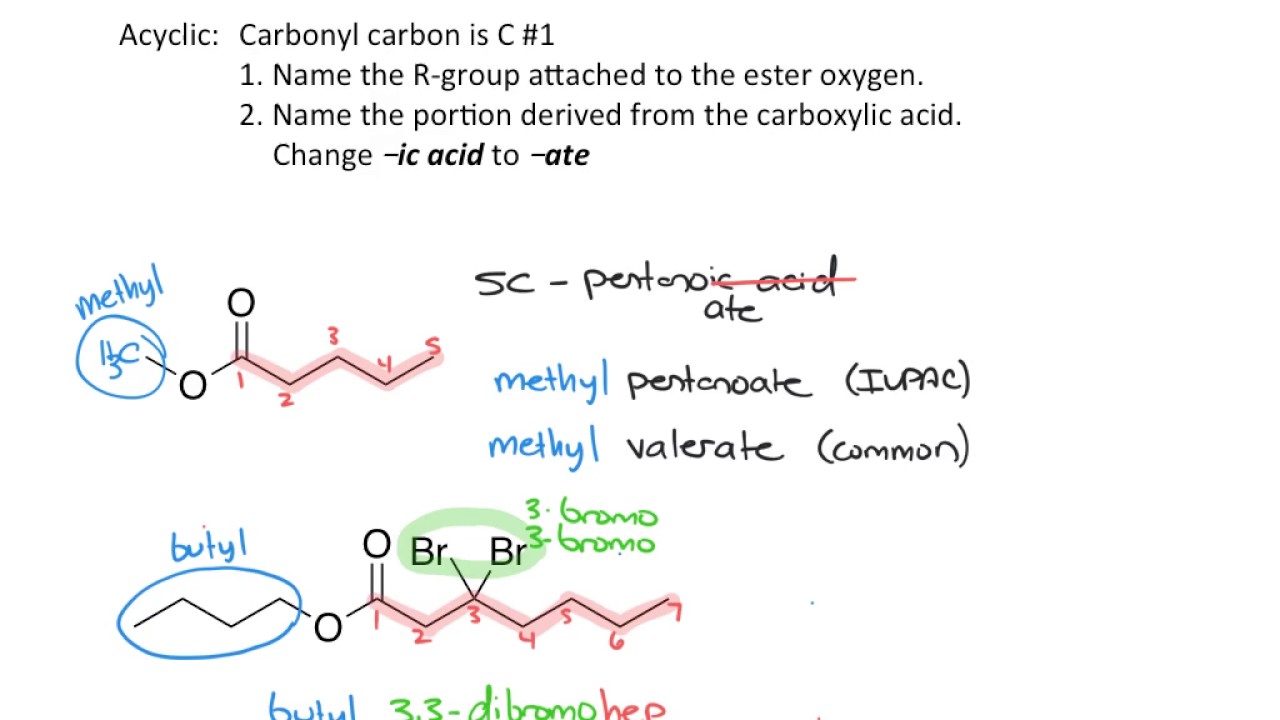
- 📚 Naming esters is based on the parent carboxylic acid, with the suffix '-oic acid' replaced by '-oate'.
- 🔑 The ester's R' group (methyl group) is named as a prefix to the parent chain name.
- 🔢 The numbering of the parent chain is done to give the carbonyl carbon the lowest number.
- 🔄 In cases where the ester has substituents, they are named with prefixes like 'methyl' or 'ethyl'.
- 📈 Multiple ester groups in a molecule require the prefix 'di-', indicating two ester groups.
- 🌿 When the parent chain is a benzene ring, the ester is named as 'benzoate', retaining the parent name.
- 📚 The priority of functional groups determines the name; esters typically take precedence over aldehydes.
- 📘 The video offers additional resources for learning organic chemistry, including an ebook and online tutoring.
Q & A
What is an ester in the context of organic chemistry?
-An ester is a molecule that contains an R group attached to an acyl or carbonyl group (a carbon double bonded to oxygen), which is then attached to a single oxygen, and another R group (R') that can be the same or different from the original R group.
How is an ester different from a carboxylic acid or an aldehyde?
-An ester is different from a carboxylic acid, which has a C=O double bond and a single bond to OH, and an aldehyde, which has a C=O double bond and a single bond to H. An ester specifically has an R group and an acyl group attached to an oxygen.
Can you explain the process of forming an ester from a carboxylic acid and an alcohol?
-To form an ester, you remove the -OH group (hydroxyl group) from the carboxylic acid and the hydrogen from the -OH group of the alcohol, then join the two molecules together.
What is the naming convention for esters?
-Esters are named as if they are carboxylic acids, with the suffix '-oic acid' replaced by '-oate'. Any substituents on the ester group are indicated with prefixes.
How do you name an ester with a methyl group attached to the oxygen?
-You prefix the name with 'methyl' to indicate the methyl group attached to the oxygen, and then follow the naming convention for the rest of the ester.
What is the name of an ester derived from a four-carbon parent chain and an ethyl substituent?
-The ester would be named 'ethyl butanoate', following the naming convention where 'butanoic' comes from the four-carbon parent chain and 'ethyl' is the prefix for the two-carbon substituent.
How do you handle multiple ester groups in a single molecule?
-When there are multiple ester groups, you add the prefix 'di-' for two esters, and the suffix '-oate' becomes '-di-oate'. The substituents are named individually with prefixes.
What is the difference between a benzyl and a phenyl substituent?
-A benzyl substituent is a benzene ring with an additional CH2 group, while a phenyl substituent is just the benzene ring itself.
How is an ester with a benzene ring named?
-The ester is named by retaining the parent name of the benzene ring ('benzoate') and then adding the substituent name and the ester suffix.
What is the final name of an ester with a three-carbon parent chain and two methyl substituents on the ester group?
-The ester would be named 'dimethyl propanedioate', indicating two methyl substituents on a three-carbon parent chain with two ester groups.
What is the significance of the number one being understood and not included in the name of an ester?
-In ester nomenclature, the number one is understood to be the position of the terminal functional group, so it is not explicitly stated in the name.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)