Charles’s Law Experiment #JayChem
Summary
TLDRThis video script details a Charles' Law experiment, demonstrating the relationship between gas volume and temperature. A flask filled with air is heated in boiling water at 100°C (373.15 K), then inverted in cold water at 23°C. As the gas cools, it contracts, drawing water into the flask. The initial volume of gas is measured as 138 mL, and after cooling, the volume decreases to 111.5 mL, illustrating Charles' Law: volume is directly proportional to temperature when pressure is constant.
Takeaways
- 🔍 The experiment demonstrates Charles' Law, which relates the volume of a gas to its temperature.
- 🌡️ The initial temperature of the gas in the flask is set to 100 degrees Celsius (372 Kelvin), simulating boiling water conditions.
- 💧 The flask is inverted in cold water at 23 degrees Celsius to observe the effect of temperature decrease on gas volume.
- 📉 As the gas cools, its volume decreases, causing water to be drawn into the flask.
- 📏 The volume of water that enters the flask is measured to be 26.5 milliliters, representing the reduction in gas volume.
- 🔄 The initial volume of gas in the flask is assumed to be the full volume of the flask, which is 138 milliliters.
- ➖ To find the new volume of the gas after cooling (V2), subtract the volume of water drawn in from the initial volume (V1).
- 📐 V2 is calculated to be approximately 111.5 milliliters, indicating the volume change due to temperature decrease.
- 🔄 Charles' Law states that the ratio of initial volume to initial temperature should equal the ratio of final volume to final temperature.
- 📝 The experiment aims to verify Charles' Law by comparing the calculated V2 with the observed V2 from the data collection.
Q & A
What is the purpose of the Charles Law experiment described in the transcript?
-The purpose of the experiment is to observe the relationship between the volume of a gas and its temperature, as described by Charles' Law, which states that the volume of a gas is directly proportional to its temperature when pressure is held constant.
What is the initial temperature (T1) of the gas inside the flask when it is placed in boiling water?
-The initial temperature (T1) of the gas inside the flask is 100 degrees Celsius, which is also converted to Kelvin for the experiment.
How is the temperature converted from Celsius to Kelvin?
-The temperature in Kelvin is equal to the temperature in Celsius plus 273.15. So, 100 degrees Celsius is equal to 373.15 Kelvin.
What happens to the gas volume when the flask is placed in cold water?
-When the flask is placed in cold water, the gas inside cools down and contracts, leading to a decrease in volume, which causes water to be drawn into the flask.
What is the temperature of the cold water bath and how is it recorded?
-The temperature of the cold water bath is 23 degrees Celsius, and this is recorded as the second data point for temperature in the experiment.
Why does water rush into the flask when it is inverted in the cold water?
-Water rushes into the flask because the gas inside contracts as it cools, creating a vacuum that allows water to be drawn in due to atmospheric pressure.
How is the volume of the gas that has cooled and contracted measured?
-The volume of the cooled gas is measured indirectly by subtracting the volume of water that has been sucked into the flask from the total volume of the flask when it was full of hot gas.
What is the volume of water that is sucked into the flask when it is inverted in the cold water?
-The volume of water that is sucked into the flask is 26.5 milliliters.
What is the initial volume (V1) of the hot air in the flask before it is inverted in the water?
-The initial volume (V1) of the hot air in the flask is 138 milliliters, as measured by pouring the contents of the flask into a graduated cylinder.
How is the final volume (V2) of the gas calculated?
-The final volume (V2) of the gas is calculated by subtracting the volume of water sucked into the flask (26.5 milliliters) from the initial volume of the hot air (138 milliliters), resulting in approximately 111.5 milliliters.
What does Charles' Law state about the relationship between the volume and temperature of a gas?
-Charles' Law states that for a given mass of gas at constant pressure, the volume of the gas is directly proportional to its thermodynamic temperature (measured in Kelvin).
How is the validity of Charles' Law tested in this experiment?
-The validity of Charles' Law is tested by comparing the calculated final volume (V2) based on the initial volume (V1) and temperature change with the actual measured final volume (V2) from the experiment.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
CHARLES' LAW | Animation
Charles' Law Experiment | Gas Laws | Class 9 | CBSE | NCERT | ICSE
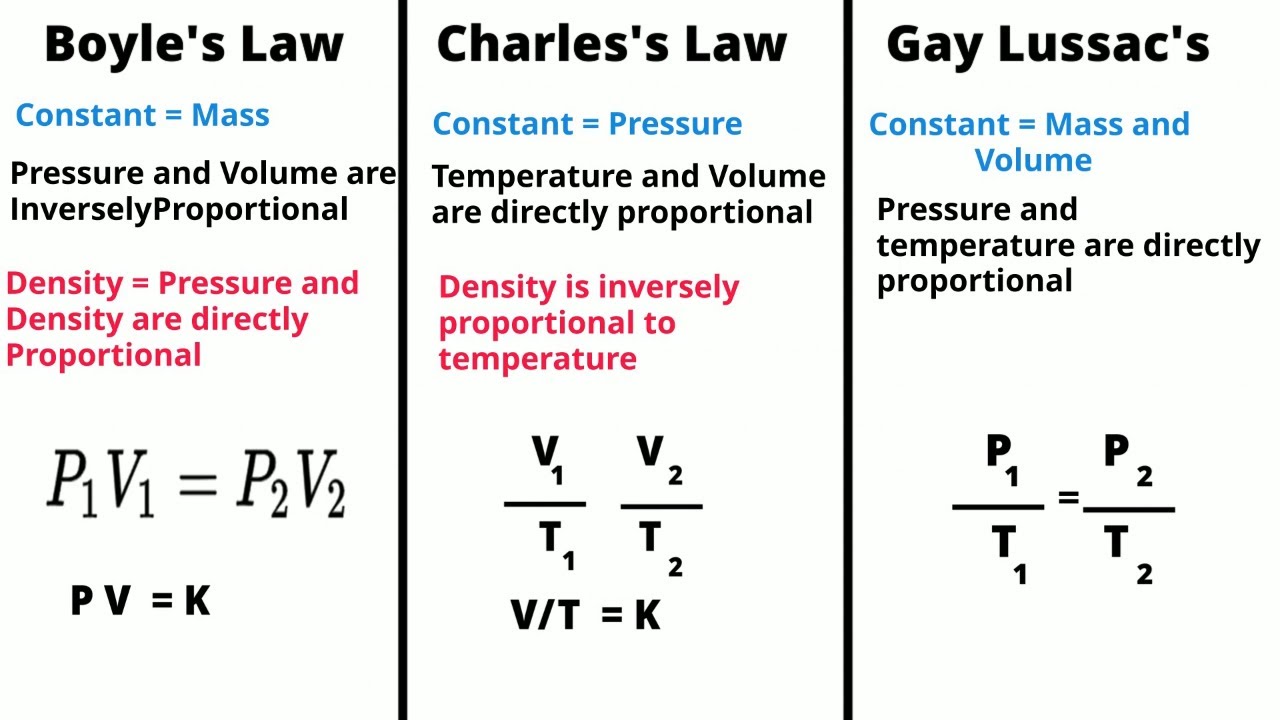
Gas Laws-Boyle's-Charles's-Gay Lussac's

פיזיקה למתכנתים הרצאה 7 חלק 2 - משוואת הגז האידיאלי

IPA FISIKA : Gas Ideal Hukum Charles, Hukum Gay Lussac dan Hukum Boyle (Simulasi)

Teori Kinetik Gas • Part 1: Sifat Gas Ideal & Hukum-Hukum Gas Ideal
5.0 / 5 (0 votes)