פיזיקה למתכנתים הרצאה 7 חלק 2 - משוואת הגז האידיאלי
Summary
TLDRThis transcript covers the development of the ideal gas law, describing the relationship between the volume, pressure, and temperature of gases. The lecture highlights key experiments by Boyle, Charles, and Guy-Lussac, each discovering fundamental principles about gases under different conditions. Boyle's law links pressure and volume, Charles' law connects volume and temperature, and Guy-Lussac's law explores pressure and temperature. The culmination of these findings leads to the ideal gas equation, which relates pressure, volume, and temperature in a simplified model, though with some limitations for more complex gases.
Takeaways
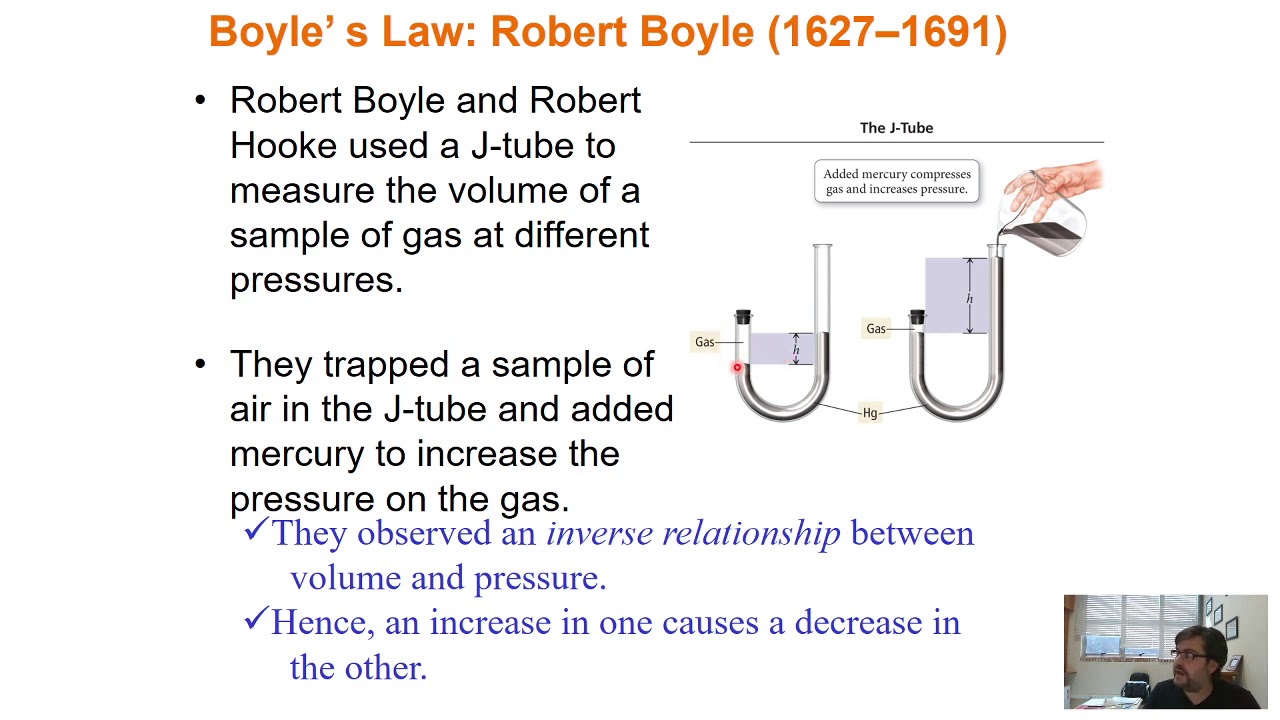
- 😀 Boyle's experiment demonstrated that pressure and volume are inversely related in gases, showing that their product remains constant.
- 😀 Charles' experiment showed that the volume of a gas is directly proportional to its temperature when pressure is held constant.
- 😀 Gay-Lussac discovered that the pressure of a gas is directly proportional to its temperature when volume is constant.
- 😀 Gay-Lussac's discovery led to the understanding that pressure increases with temperature, leading to the introduction of the Kelvin scale for temperature.
- 😀 The concept of absolute zero emerged, where there would be no pressure in a gas, corresponding to a temperature of -273.15°C.
- 😀 The Kelvin scale of temperature was introduced, where 0 K corresponds to absolute zero and is calculated as the Celsius temperature plus 273.15.
- 😀 The Ideal Gas Law was formulated by Emile Clapeyron, expressing the relationship between pressure, volume, and temperature in gases as PV = K * T.
- 😀 The Ideal Gas Equation is applicable to any gas under a wide range of conditions, as long as temperature is measured in Kelvin.
- 😀 The constant K in the Ideal Gas Law depends on the amount of gas and its type, affecting the behavior of the gas in the equation.
- 😀 Although the Ideal Gas Law is a good approximation for many gases, it is not perfectly accurate, especially for complex gases or under extreme conditions.
Q & A
What is the ideal gas equation?
-The ideal gas equation states that for any gas, the product of pressure (P) and volume (V) is proportional to the temperature (T) of the gas, with a constant K. This can be written as P * V = K * T, where K is a constant that depends on the amount and type of gas.
What was Boyle's contribution to understanding gas behavior?
-Boyle conducted an experiment where he discovered that the volume of a gas decreases as the pressure increases, while keeping the temperature constant. This led to Boyle's law, which states that pressure and volume are inversely proportional (P * V = constant).
How did Charles contribute to the understanding of gas behavior?
-Charles discovered that the volume of a gas increases as its temperature increases, while keeping the pressure constant. This is known as Charles' law, which can be written as V = constant * T when pressure is constant.
What did Gay-Lussac discover about gas pressure and temperature?
-Gay-Lussac found that when the volume of a gas is kept constant, the pressure of the gas increases with an increase in temperature. This is described by Gay-Lussac's law, which states that pressure is directly proportional to temperature at constant volume.
Why did Gay-Lussac's discovery about temperature and pressure require accurate temperature measurement tools?
-Gay-Lussac's experiment required accurate temperature measurements because temperature plays a crucial role in determining the pressure of a gas. At the time of Boyle's experiment, accurate temperature measurement tools had not yet been developed, making Gay-Lussac's experiment possible only later.
What did Gay-Lussac observe regarding the pressure of a gas at very low temperatures?
-Gay-Lussac observed that if the temperature of a gas decreases, its pressure also decreases. He noted that the pressure of the gas reached zero at a temperature of -273.15°C, which led to the concept of absolute zero, where a gas exerts no pressure.
Why are temperatures measured in Kelvin rather than Celsius in the ideal gas law?
-Temperatures are measured in Kelvin because the ideal gas law assumes that temperature cannot go below absolute zero, which is 0 K. In Celsius, temperatures can go negative, which would require adjustments to the ideal gas equation, while Kelvin provides a more accurate representation for the law.
What is the significance of the constant K in the ideal gas equation?
-The constant K in the ideal gas equation depends on the amount of gas and its type. It represents a proportionality constant that links pressure, volume, and temperature in the ideal gas law.
How did the development of scientific tools influence the discoveries about gases?
-The development of scientific tools, such as barometers, manometers, and thermometers, was crucial for experiments by Boyle, Charles, and Gay-Lussac. These tools allowed scientists to measure pressure, volume, and temperature accurately, leading to the formulation of gas laws.
Why is the ideal gas law considered an approximation?
-The ideal gas law is considered an approximation because it assumes that gases behave perfectly, which is not always the case, especially at high pressures or low temperatures. Real gases deviate from ideal behavior under certain conditions, requiring adjustments to the equation.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)