Chemicals of Life - Nucleic Acids - Post 16 Biology (A Level, Pre-U, IB, AP Bio)
Summary
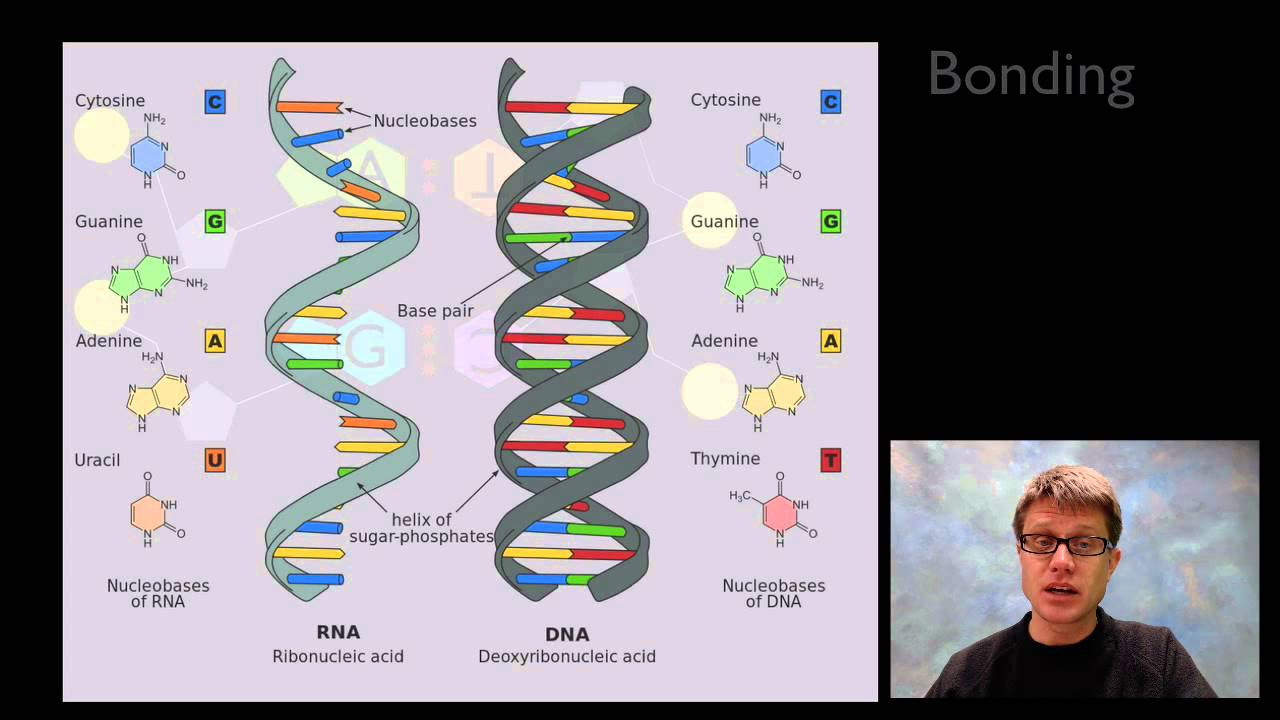
TLDRThis script introduces nucleic acids, comprising DNA, RNA, and ATP. DNA is a double-stranded molecule with a double helix structure, made of nucleotides containing a phosphate group, deoxyribose sugar, and nitrogenous bases (cytosine, thymine, adenine, guanine). The strands run antiparallel, with bases pairing complementarily (A-T, C-G). RNA is single-stranded, with ribose sugar and uracil instead of thymine. ATP, a nucleotide with adenine, ribose, and three phosphate groups, serves as the cell's energy currency, releasing energy upon bond breakage.
Takeaways
- 🧬 Nucleic acids are composed of nucleotides, which include a phosphate group, a pentose sugar, and a nitrogenous base.
- 🌟 DNA (Deoxyribonucleic acid) is a well-known nucleic acid made up of nucleotides with four different bases: adenine, thymine, cytosine, and guanine.
- 🔍 The DNA structure is a double helix, resembling a twisted ladder, with each strand being a polymer of nucleotides.
- 🔗 DNA nucleotides are linked by phosphodiester bonds, formed through condensation reactions that remove water.
- 🌐 DNA strands are antiparallel, with one strand running in the opposite direction to the other, facilitating base pairing.
- 🤝 Complementary base pairing in DNA involves adenine pairing with thymine and cytosine pairing with guanine.
- 🧵 RNA (Ribonucleic acid) is structurally similar to DNA but has three key differences: it's single-stranded, uses ribose instead of deoxyribose, and contains uracil instead of thymine.
- 🔋 ATP (Adenosine triphosphate) is a nucleotide that contains three phosphate groups and is crucial for cellular energy transfer.
- ⚡ Breaking the bond between the last two phosphates in ATP releases energy, which cells use for metabolic processes.
- 🔄 ATP is often referred to as the 'energy currency' of the cell, highlighting its role in providing energy for various cellular functions.
Q & A
What are nucleic acids composed of?
-Nucleic acids are composed of nucleotides, which consist of a phosphate group, a sugar (pentose), and a nitrogenous base.
What are the three types of nucleic acids mentioned in the script?
-The three types of nucleic acids mentioned are deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and adenosine triphosphate (ATP).
What is the structure of DNA?
-DNA has a double helix structure, which is like a twisted ladder, with each ladder rung being a pair of nucleotides.
What are the four different nucleotides that make up DNA?
-The four different nucleotides that make up DNA are adenine (A), thymine (T), cytosine (C), and guanine (G).
What is the sugar component of DNA nucleotides?
-The sugar component of DNA nucleotides is deoxyribose.
How are nucleotides linked together in DNA?
-Nucleotides are linked together in DNA by phosphodiester bonds, which are formed through condensation reactions that remove water.
What is the significance of the antiparallel arrangement of DNA strands?
-The antiparallel arrangement of DNA strands means that one strand runs in one direction and the other in the opposite direction, allowing for complementary base pairing.
What is complementary base pairing in DNA?
-Complementary base pairing in DNA is the specific pairing of nucleotide bases where adenine (A) pairs with thymine (T), and cytosine (C) pairs with guanine (G).
How does RNA differ from DNA?
-RNA differs from DNA in that it is single-stranded, it contains the sugar ribose instead of deoxyribose, and it contains uracil instead of thymine.
What is ATP and why is it important for cells?
-ATP, or adenosine triphosphate, is a nucleotide with adenine as its base, ribose as its sugar, and three phosphate groups. It is crucial for cells because breaking the bond between the last two phosphates releases energy that cells use for metabolic processes.
How many hydrogen bonds are there between adenine and thymine in DNA?
-There are two hydrogen bonds between adenine and thymine in DNA.
How many hydrogen bonds are there between cytosine and guanine in DNA?
-There are three hydrogen bonds between cytosine and guanine in DNA.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)