Intermolecular Forces | Chemistry
Summary
TLDRThis educational script explains intermolecular forces, which are the attractive or repulsive forces between molecules. It distinguishes between these forces and intramolecular forces found in metals. The script covers three types of intermolecular forces: hydrogen bonding, dipole-dipole forces, and London dispersion forces. It also highlights the relationship between boiling points and intermolecular forces, noting that stronger forces correlate with higher boiling points. The strongest force is hydrogen bonding, while the weakest is London dispersion force.
Takeaways
- 💧 **Intermolecular Forces Defined**: Attractive or repulsive forces between molecules of a compound.
- 🔗 **Electrostatic Nature**: All intermolecular forces are electrostatic and natural, resulting from the attraction or repulsion between opposite or similar charges.
- 💧 **Types of Forces**: Three main types are hydrogen bonding, dipole-dipole forces, and London dispersion forces.
- 💧 **Presence in Non-Metals**: Intermolecular forces exist only in non-metals, not in metals where metallic bonds prevail.
- 🔗 **Hydrogen Bonding**: The strongest intermolecular force, occurring when hydrogen is bonded to a strongly electronegative element.
- 🌡️ **Boiling Points**: Directly proportional to intermolecular forces; compounds with stronger forces have higher boiling points.
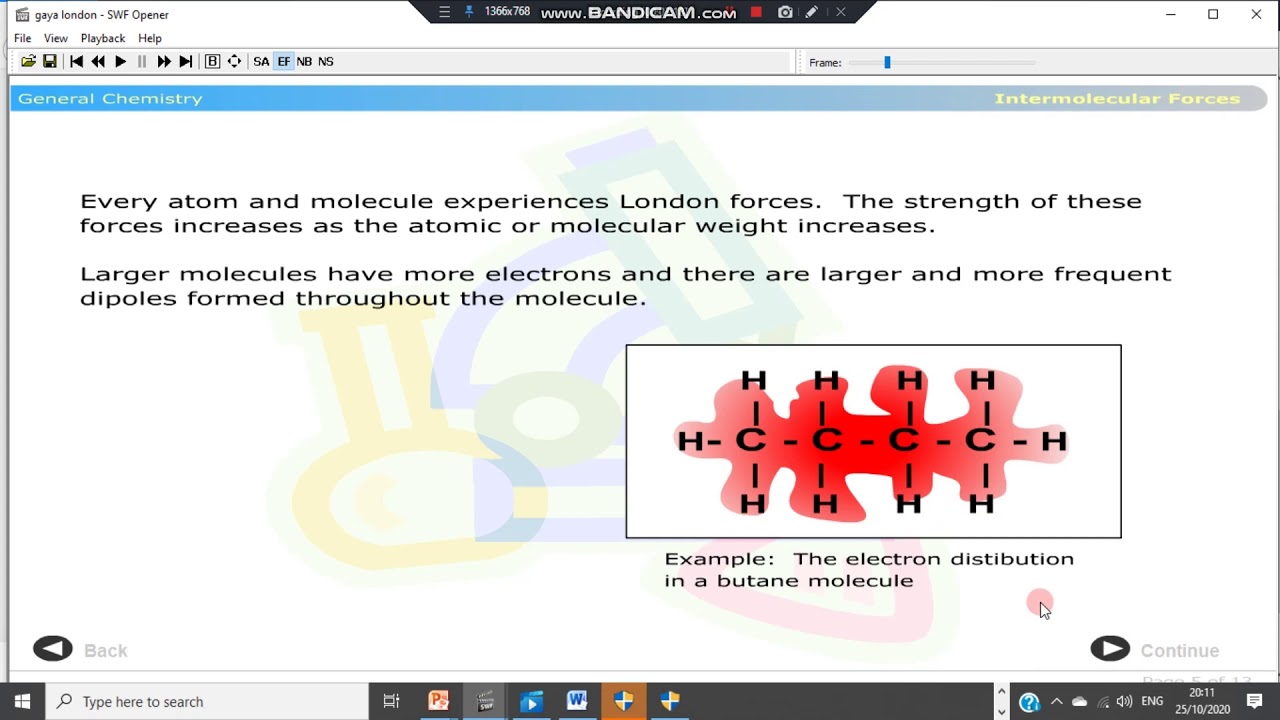
- 💧 **London Dispersion Forces**: The weakest intermolecular force, increasing with the number of electrons in a molecule.
- 🔬 **Forces in Metals**: Metals contain intramolecular forces like metallic bonds rather than intermolecular forces.
- 🌡️ **Boiling Point Examples**: Water (100°C) has strong forces, whereas methane (-162°C) has weak forces, illustrating the strength of London dispersion forces.
- 📚 **Further Learning**: Encouragement to watch more lectures for a deeper understanding of intermolecular forces.
Q & A
What are intermolecular forces?
-Intermolecular forces are the attractive or repulsive forces that exist between molecules of a compound. They are electrostatic and natural in nature.
How do intermolecular forces differ from intramolecular forces?
-Intermolecular forces exist between molecules, whereas intramolecular forces, such as chemical bonds like metallic bonds, exist within a molecule.
Are intermolecular forces present in metals?
-No, intermolecular forces are not present in metals. Metals contain intramolecular forces like metallic bonds instead.
What types of intermolecular forces are typically studied at the college level?
-At the college level, three types of intermolecular forces are studied: hydrogen bonding, dipole-dipole forces, and London dispersion forces.
Which compounds exhibit hydrogen bonding?
-Hydrogen bonding is present in compounds like water (H2O), hydrogen fluoride (HF), and ammonia (NH3).
Between which types of molecules do dipole-dipole forces exist?
-Dipole-dipole forces exist between two polar molecules, such as HCl and sulfur dioxide.
What is the role of London dispersion forces in non-polar molecules?
-London dispersion forces exist between non-polar molecules like hydrogen gas, fluorine gas, chlorine gas, and oxygen gas.
How does the strength of intermolecular forces relate to boiling points?
-The boiling point of a compound is directly proportional to the strength of its intermolecular forces. Compounds with strong intermolecular forces have higher boiling points.
What is the strongest intermolecular force?
-Hydrogen bonding is the strongest intermolecular force. It occurs when hydrogen is bonded to a strongly electronegative element.
What is the weakest intermolecular force?
-London dispersion forces are considered the weakest intermolecular forces. They increase in strength with the number of electrons in a molecule.
How do intermolecular forces affect the ease of separating molecules?
-Strong intermolecular forces require more thermal energy to separate molecules, while weak intermolecular forces require less.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Intermolecular vs Intramolecular forces Grade 11 Chemistry

Intramolecular vs. Intermolecular forces - London Dispersion, Dipole-Dipole, Ion-Dipole forces -Chem

Ikatan Kimia (9) | Gaya Antar Molekul | Ikatan Antar Molekul |Kimia kelas 10

How to identify intermolecular forces?

What are Intermolecular Forces?
Gaya antar molekul ; dipole dipole, gaya london dan ikatan hidrogen
5.0 / 5 (0 votes)