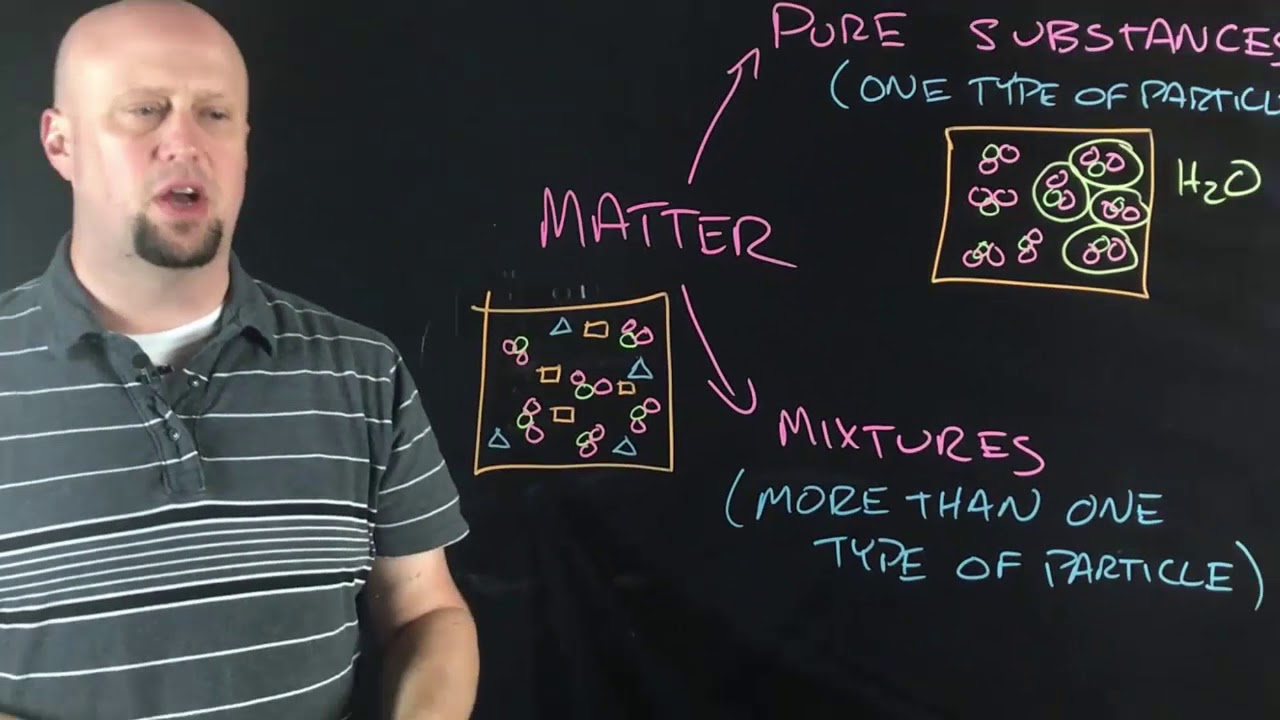
Molecules and compounds
Summary
TLDRIn this educational video, the focus is on molecules and compounds, essential building blocks of matter. The script explains that all matter is composed of atoms, which in turn are made up of protons, neutrons, and electrons. It delves into the composition of living organisms, highlighting the significance of carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur. The video introduces the concept of molecules formed by atoms bonding together, distinguishing between diatomic and non-diatomic molecules, exemplified by oxygen gas and water, respectively. It also touches on molecular models like ball and stick, and space-filling, emphasizing how molecular shape influences properties. The script concludes with a discussion on compounds, formed by different elements bonding, and the importance of understanding chemical formulas for elements like water, H2O.
Takeaways
- 🌐 Atoms are the basic building blocks of matter, consisting of protons, neutrons, and electrons.
- 🌿 Living organisms, including humans, are composed of various types of atoms, primarily carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur.
- 🔬 Molecules are formed when two or more atoms bond together, which can be of the same or different elements.
- 💧 Examples of molecules include oxygen (O2), hydrogen (H2), and water (H2O), with water being a compound made of two hydrogen atoms and one oxygen atom.
- 🔬 Diatomic molecules consist of exactly two atoms, such as O2, H2, N2, and Cl2.
- 🌐 Non-diatomic molecules have more than two atoms, like water (H2O), which has three atoms in total.
- 🔍 Molecular models, such as ball and stick or space-filling models, are used to visualize and understand the structure of molecules.
- 📏 The arrangement of atoms within a molecule, such as angles or straight lines, affects the molecule's properties and behavior.
- 🧪 Compounds are formed when atoms of different elements bond together, creating a new substance with distinct properties.
- 🔢 Chemical formulas represent the composition of molecules, showing the number and types of atoms present, and can be used for molecular math to determine the total number of atoms in a compound.
Q & A
What are the basic building blocks of matter?
-The basic building blocks of matter are molecules and compounds, which are made up of atoms.
What are the three subatomic particles found in an atom?
-The three subatomic particles found in an atom are protons, neutrons, and electrons.
How do protons in an atom determine its properties?
-Protons determine the type of atom and its properties because they carry a positive charge and their number defines the atomic number of the element.
What are the main elements that make up living organisms?
-The main elements that make up living organisms are carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur.
How do molecules form?
-Molecules form when two or more atoms bond together, which can be atoms of the same kind or different kinds.
What is a diatomic molecule and provide some examples?
-A diatomic molecule consists of exactly two atoms. Examples include oxygen (O2), hydrogen (H2), nitrogen (N2), and chlorine (Cl2).
What is a non-diatomic molecule and how does it differ from a diatomic molecule?
-A non-diatomic molecule consists of more than two atoms. It differs from a diatomic molecule in that it has a greater number of atoms, which can affect its properties.
What are the two types of molecular models mentioned in the script?
-The two types of molecular models mentioned are the ball and stick model, where atoms are represented as spheres and bonds as sticks, and the space-filling model, where atoms are represented as intersecting spheres.
How does the arrangement of atoms in a molecule affect its properties?
-The arrangement of atoms in a molecule affects its properties by influencing its shape, which in turn can determine its reactivity, polarity, and other chemical behaviors.
What is a compound and how is it different from a molecule?
-A compound is a substance formed when atoms of different elements bond together, often resulting in molecules with more complex structures than simple diatomic or non-diatomic molecules.
How can you determine the number of atoms in a compound using its chemical formula?
-You can determine the number of atoms in a compound by counting the atoms indicated by the chemical formula, where numbers subscript to the right of an element symbol indicate the quantity of that atom in the molecule.
Why is it important to keep notes organized when learning about molecules and compounds?
-Keeping notes organized is important because it helps in understanding the complex relationships between atoms, molecules, and compounds, and it sets up the learner for success in science by promoting clarity and retention of information.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Perbedaan Atom, Unsur, Senyawa, Molekul dan Ion | KIMIA KELAS 10

Mengenal Unsur, Senyawa dan Campuran

1) Atoms compounds elements and molecules gr 10

Difference between an Atom, a Molecule and a Compound

Classification of matter | Structure and properties of matter | High school chemistry | Khan Academy
Classification pt 1 Pure Substances
5.0 / 5 (0 votes)