A Level Chemistry Revision "Le Chatelier's Principle and Pressure"
Summary
TLDRThis video from the 'Free Science' series explores Le Chatelier's Principle, focusing on its application to reversible reactions involving gases. It explains how changes in pressure can shift the equilibrium of reactions, using the Haber process as an example. The video illustrates that increasing pressure favors the side with fewer gas moles, while decreasing pressure favors the side with more moles. It also clarifies that equilibrium is only affected by pressure if there's a difference in the total number of moles on each side of the reaction. The lesson concludes with the impact of pressure on the color change in the dinitrogen tetroxide to nitrogen dioxide reaction, emphasizing the principle's practical implications.
Takeaways
- 🔍 Le Chatelier's Principle states that when an external change is applied to a system at equilibrium, the equilibrium adjusts to minimize the effect of that change.
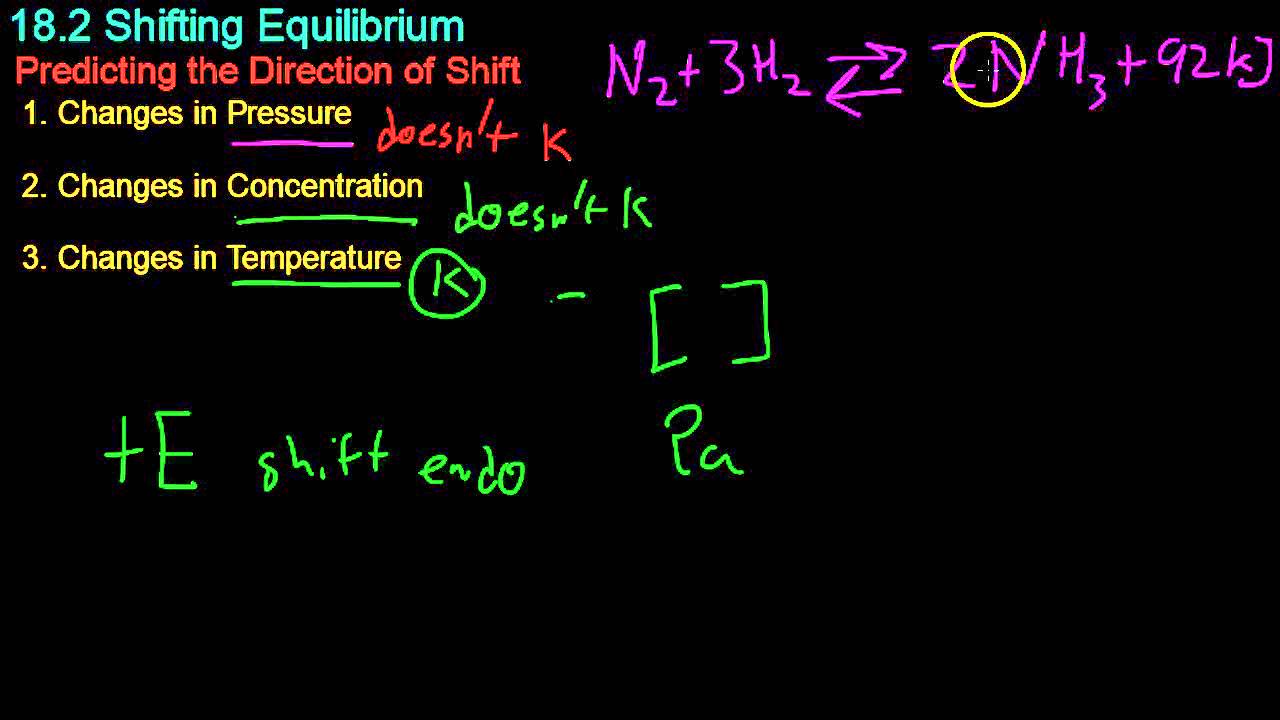
- 📚 The video focuses on applying Le Chatelier's Principle to reversible reactions involving gases and how changes in pressure affect the equilibrium.
- 🌡 Gas pressure is directly proportional to the number of moles of gas present, which is a critical concept for understanding how pressure changes impact equilibrium.
- 🔄 The Haber process is used as an example of a reversible reaction where nitrogen and hydrogen gases combine to form ammonia, and vice versa.
- 📉 Increasing the pressure in a reversible reaction will shift the equilibrium towards the side with fewer moles of gas to reduce the pressure.
- 📈 Conversely, decreasing the pressure will shift the equilibrium towards the side with more moles of gas, increasing the pressure.
- 📚 Another example given is the reversible reaction between dinitrogen tetroxide and nitrogen dioxide, where increasing pressure shifts the equilibrium to form more colorless dinitrogen tetroxide.
- 🏳 The position of equilibrium is only affected by pressure if there is a difference in the total number of moles of gas on either side of the reaction equation.
- 🔄 In the case of the reaction between hydrogen and bromine to form hydrogen bromide, since both sides have the same number of moles, pressure changes do not affect the equilibrium position.
- 📝 The video script encourages viewers to pause and apply Le Chatelier's Principle to predict the effects of pressure changes on equilibrium in different reactions.
- 🎥 The video concludes with a teaser for the next lesson, which will explore the impact of temperature on reversible reactions.
Q & A
What is Le Chatelier's principle?
-Le Chatelier's principle states that when an external change is applied to a system at equilibrium, the equilibrium moves in the direction that reduces the effect of that change.
How does Le Chatelier's principle apply to reversible reactions involving gases?
-Le Chatelier's principle can be applied to reversible reactions involving gases by changing the position of the equilibrium through changes in pressure, as gas pressure is proportional to the number of moles of gas present.
What is the Haber process mentioned in the script?
-The Haber process is a reversible reaction where nitrogen and hydrogen gases react to form ammonia gas.
How does the number of moles of gas affect the equilibrium position in the Haber process?
-In the Haber process, there are four moles of reactants (1 mole of nitrogen and 3 moles of hydrogen) and two moles of product (ammonia). If the equilibrium is entirely on the reactant side, the pressure would be twice as great as if it were entirely on the product side.
What happens to the equilibrium position if the pressure is increased in the Haber process?
-If the pressure is increased, the equilibrium will move in the direction that reduces the pressure, which is towards the right, producing more ammonia.
What would be the effect on the equilibrium if the pressure is reduced in the Haber process?
-If the pressure is reduced, the equilibrium will move in the direction that increases the pressure, which is towards the left, converting more ammonia back into nitrogen and hydrogen.
What is the reaction involving dinitrogen tetroxide and nitrogen dioxide?
-The reaction involves dinitrogen tetroxide, a colorless gas, forming nitrogen dioxide, which is brown.
How does increasing pressure affect the equilibrium position in the reaction between dinitrogen tetroxide and nitrogen dioxide?
-Increasing the pressure will cause the equilibrium to move towards the left, reducing the pressure by converting nitrogen dioxide back into dinitrogen tetroxide, making the reaction mix less brown.
Why does the position of equilibrium not change with pressure in some reactions?
-The position of equilibrium is only affected by pressure if the total number of moles is different on either side of the equation. If the moles of gas are the same on both sides, changing the pressure has no effect on the equilibrium position.
What is the example given in the script for a reaction where pressure does not affect the equilibrium position?
-The example given is the reaction between hydrogen and bromine to form hydrogen bromide, where there are two moles of gas on both the reactant and product sides.
What will be the focus of the next video in the series?
-The next video will focus on the effect of temperature on reversible reactions.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)