Oxidative phosphorylation Animation - Formation of ATP & sites of ATP synthesis
Summary
TLDRThis script delves into oxidative phosphorylation, a vital cellular process for ATP production. It highlights three key ATP-synthesizing sites within the electron transport chain: between NAD and Coenzyme Q, Coenzyme Q and Cytochrome C, and Cytochrome C and Oxygen. The script explains the role of the F0F1 ATPase enzyme, commonly known as ATP synthase, in harnessing the proton gradient across the mitochondrial membrane to synthesize ATP. The mechanism of ATP synthesis is further elucidated through the binding change mechanism proposed by Paul Boyer, emphasizing the molecular motor function of the F1 complex and its gamma subunit's pivotal role in the process.
Takeaways
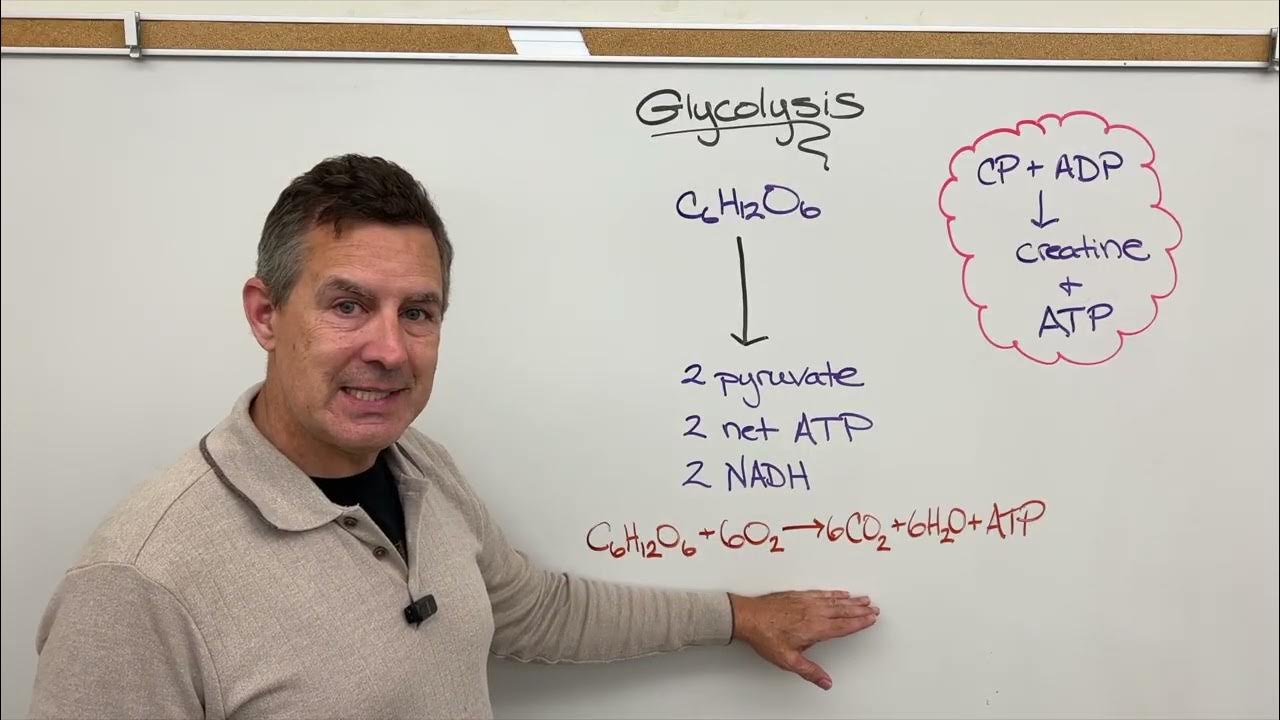
- 🌟 Oxidative phosphorylation is the process of ATP formation coupled with the transfer of electrons through the electron transport chain.
- 🔋 There are three main ATP synthesizing sites in the electron transport chain: between NADH and Coenzyme Q (Complex I), Coenzyme Q and Cytochrome C (Complex III), and Cytochrome C and Oxygen (Complex IV).
- ❌ Complex II does not contribute to ATP synthesis, highlighting the specificity of ATP production sites within the electron transport chain.
- 💡 The energy released at Complex I and Complex III is utilized to synthesize one ATP molecule at each site through the movement of hydrogen ions.
- 🔄 Complex IV (Site III) is unique as it only synthesizes half an ATP molecule due to the pumping of two hydrogen ions into the intermembrane space.
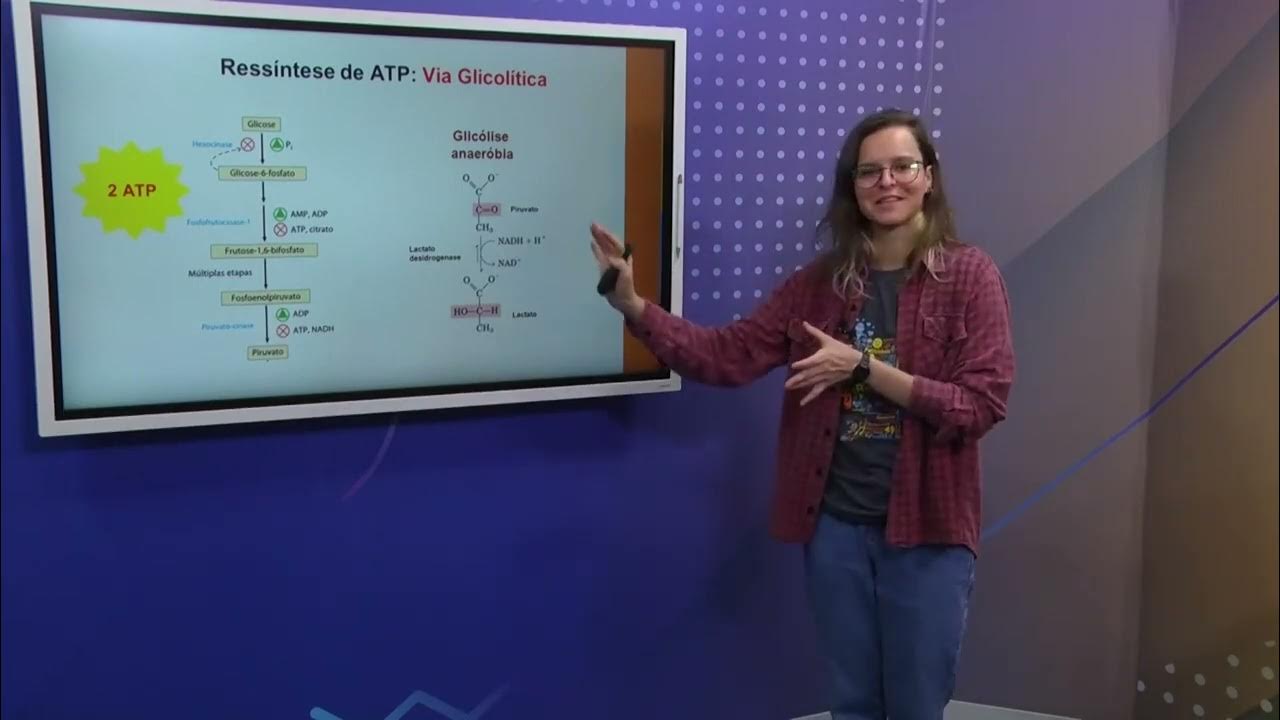
- 🔢 The entry of one NADH molecule into the respiratory chain results in the production of 2.5 ATP molecules, while one FADH2 molecule yields 1.5 ATP molecules.
- 🛠 The chemiosmotic theory by Peter Mitchell (1961) explains the coupling of oxidation and phosphorylation through a proton gradient across the mitochondrial membrane.
- 🔧 ATP synthase, also known as Complex V, is a molecular motor embedded in the inner mitochondrial membrane, consisting of F0 and F1 subcomplexes.
- 🔁 The F0 subcomplex forms a proton channel and is responsible for the rotation that drives ATP synthesis, while the F1 subcomplex contains the catalytic site for ATP production.
- 🔄 The gamma subunit of the F1 complex rotates due to the proton flow through F0, causing conformational changes necessary for ATP synthesis in the beta subunits.
- 🔒 Respiratory control refers to the tight coupling of electron flow and ATP synthesis, ensuring oxygen consumption is dependent on the availability of ADP.
- 🔄 The binding change mechanism, proposed by Paul Boyer, describes the process of ATP production involving the re-entry of protons and the subsequent rotation and conformational changes in the F1 complex.
Q & A
What is oxidative phosphorylation?
-Oxidative phosphorylation is the process where ATP is formed during the transfer of electrons through the electron transport chain, coupled with the phosphorylation of ADP by an enzyme, utilizing the energy produced.
How many ATP synthesizing sites are there in the electron transport chain?
-There are three ATP synthesizing sites in the electron transport chain: between NAD and Coenzyme Q (Complex I), between Coenzyme Q and Cytochrome C (Complex III), and between Cytochrome C and Oxygen (Complex IV).
Why does Complex II not produce any ATP?
-Complex II does not pump protons into the intermembrane space, which is necessary for the generation of the electrochemical gradient that drives ATP synthesis.
How many ATP molecules are synthesized at Complex I and Complex III?
-At both Complex I and Complex III, one ATP molecule is synthesized per site due to the proton gradient created by the pumping of four hydrogen ions.
What is the role of Complex IV in ATP synthesis?
-Complex IV, also known as site three, is responsible for pumping two hydrogen ions into the intermembrane space, which results in the synthesis of only half an ATP molecule when they are pumped back into the mitochondrial matrix.
How many ATP molecules are produced when one NADH molecule enters the respiratory chain?
-When one NADH molecule enters the respiratory chain, it produces 2.5 molecules of ATP, considering the energy liberation and proton gradient across the complexes.
What is the significance of the Kimmi osmotic theory in oxidative phosphorylation?
-The Kimmi osmotic theory, proposed by Peter Mitchell, explains the coupling between the oxidation of reducing equivalents and the phosphorylation of ADP into ATP through a proton gradient across the mitochondrial membrane.
What are the two sub-complexes of ATP synthase?
-The two sub-complexes of ATP synthase are Fo (also known as F naught), which is hydrophobic and forms a proton channel, and F1 (also known as F subunit), which is hydrophilic and contains the catalytic site for ATP synthesis.
How does the rotation of the Fo complex and the gamma subunit of F1 complex contribute to ATP synthesis?
-The flow of protons through Fo causes the rotation of the Fo complex along with the gamma subunit of F1, which in turn causes conformational changes in the beta subunits of F1, leading to ATP synthesis.
What is the respiratory control, and how does it relate to ADP availability?
-Respiratory control is the tight coupling of electron flow and ATP synthesis in mitochondria, ensuring that oxygen consumption depends on the availability of ADP. It adjusts electron flow and oxygen consumption based on the body's energy demands.
What is the binding change mechanism, and how does it explain ATP production in the F1 sub complex?
-The binding change mechanism, proposed by Paul Boyer, states that the re-entry of protons through the Fo sub complex causes rotation of the gamma subunit of F1, leading to conformational changes in the beta subunits, which is the site of ATP synthesis.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тариф5.0 / 5 (0 votes)