Why Does Everything Decay Into Lead
Summary
TLDRThis video explores the fascinating world of nuclear physics and how elements decay into lead, a stable and 'magical' element. It delves into radioactive decay chains, explaining the process of alpha and beta decay, and introduces the concept of 'magic numbers,' which contribute to an atom’s stability. The video discusses the work of Maria Goeppert Mayer and Eugene Wigner in uncovering these patterns in atomic nuclei, leading to a deeper understanding of the periodic table. It also touches on the potential discovery of new 'magic numbers' and the search for an island of stability among superheavy elements.
Takeaways
- 😀 Lead has been used in various ways throughout history, from ancient Roman wine sweetening to modern dental X-ray shielding.
- 😀 Every element beyond lead is radioactive, and they eventually decay into lead over time.
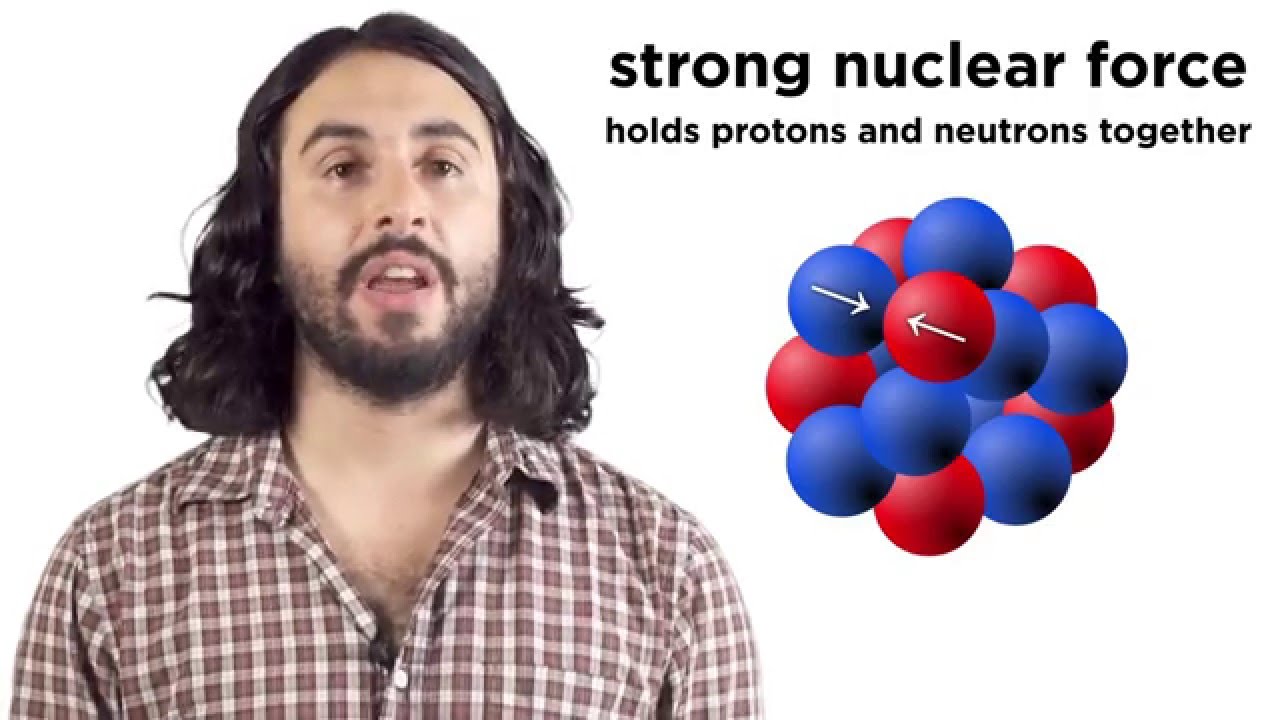
- 😀 An unstable atom's nucleus may undergo alpha or beta decay, changing the number of protons and neutrons and, ultimately, the element itself.
- 😀 Decay chains are series of transformations through alpha and beta decays, leading to stable isotopes such as lead-206, lead-207, and lead-208.
- 😀 Thorium-232 begins the thorium decay chain, ultimately decaying into stable lead-208 through a series of alpha and beta decays.
- 😀 The neptunium decay chain, which does not end in lead, involves isotopes with incredibly long half-lives, such as bismuth-209.
- 😀 Some isotopes are stable while others are radioactive due to an imbalance between protons and neutrons in the nucleus.
- 😀 The 'valley of stability' concept shows that stable isotopes have a specific ratio of protons to neutrons, with lead being the endpoint for most decay chains.
- 😀 Nuclear shell theory, proposed by Maria Goeppert Mayer, explains that certain numbers of protons or neutrons are more likely to form stable isotopes, known as 'magic numbers.'
- 😀 The stability of isotopes is not guaranteed by having a magic number, but magic numbers make stability more likely, especially in larger isotopes like lead-208.
- 😀 Beyond known elements, scientists hypothesize an 'island of stability' for superheavy elements, where isotopes might have longer half-lives thanks to magic numbers.
Q & A
Why is lead considered 'magic' in nuclear physics?
-Lead is considered 'magic' because it represents a stable end point in many decay chains, with one of its isotopes, lead-208, being doubly stable, making it more resistant to radioactivity compared to other elements. This stability is due to its specific combination of protons and neutrons, which is seen as a 'magic number' in nuclear physics.
What is the relationship between the number of protons and the stability of an isotope?
-The number of protons in an isotope determines the element. To maintain stability, the number of neutrons must balance the repulsion between protons. An imbalance leads to radioactivity as the nucleus tries to achieve stability through decay.
What are decay chains, and how do they relate to lead?
-Decay chains are sequences of radioactive decays, often involving alpha and beta decay, that occur until a stable isotope is formed. Lead is the final element in several natural decay chains, such as the thorium, actinium, and radium series, marking it as a stable end point for these processes.
What is the difference between alpha decay and beta decay?
-Alpha decay occurs when a nucleus emits an alpha particle (two protons and two neutrons), while beta decay involves the emission of a beta particle, which can either be an electron or a positron, depending on the type. Both processes change the number of protons, altering the element in question.
How does the concept of 'magic numbers' apply to nuclear physics?
-Magic numbers refer to specific numbers of protons or neutrons that lead to increased nuclear stability. These numbers, such as 2, 8, 20, 28, 50, 82, and 126, are associated with closed nuclear shells, where the protons and neutrons are tightly bound, making the atom more stable.
Who discovered the concept of 'magic numbers' in nuclear physics, and how was it recognized?
-Maria Goeppert Mayer discovered the concept of magic numbers in the 1940s. She noticed that isotopes with certain numbers of protons or neutrons were more stable than others, suggesting that these nucleons occupied specific energy levels or 'shells' within the nucleus.
What is the significance of the 'valley of stability' in nuclear physics?
-The 'valley of stability' is a region in a graph of isotopes, where stable isotopes reside. It shows the balance between protons and neutrons, with stable isotopes occurring along a particular line. As elements get heavier than lead-208, they become increasingly unstable and radioactive.
Why does tin have more stable isotopes than indium?
-Tin has 50 protons, a magic number, making its isotopes more stable. In contrast, indium, with 49 protons, does not have the same stability-enhancing magic number, which leads to fewer stable isotopes.
What is the hypothetical 'island of stability' in the periodic table?
-The 'island of stability' is a theoretical region of the periodic table where superheavy elements might exist with relatively long half-lives due to unconfirmed magic numbers. These elements would still be radioactive but would have significantly longer stability than other superheavy isotopes.
What evidence is there for new magic numbers, and why is potassium-32 significant in this context?
-In 2013, studies suggested that 32 and 34 might be magic numbers. However, a 2021 study involving potassium-32 did not show the expected size jump in the nucleus, leading scientists to conclude that 32 is not a magic number. This shows how experimental testing is crucial in confirming new magic numbers.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео

NOVA scienceNOW : 19 - Island Of Stability

Nuclear Half Life: Intro and Explanation

Stellar Nucleosynthesis Explained in 4 Minutes

I never understood why too many neutrons cause instability - until now!

Regents Chemistry Nuclear Chemistry Part 3 Radioactive Decay
Nuclear Reactions, Radioactivity, Fission and Fusion
5.0 / 5 (0 votes)
