PERGESERAN KESETIMBANGAN (ASAS LE CHATELIER) : KESETIMBANGAN KIMIA KELAS 11
Summary
TLDRThis video explains the factors that cause equilibrium shifts in chemical reactions, based on Le Chatelier's Principle. The video covers three main factors: changes in concentration, temperature, and pressure/volume. It discusses how adding or removing substances can shift the equilibrium toward products or reactants. Temperature changes impact whether the reaction is exothermic or endothermic, while pressure and volume adjustments mainly affect gas-phase reactions. Examples are given to illustrate each concept, emphasizing how equilibrium responds to external changes to maintain balance, as well as how changes in system conditions can alter the concentrations of products and reactants.
Takeaways
- 😀 Le Chatelier's Principle states that a system at equilibrium will shift to counteract an external change, aiming to reach a new equilibrium.
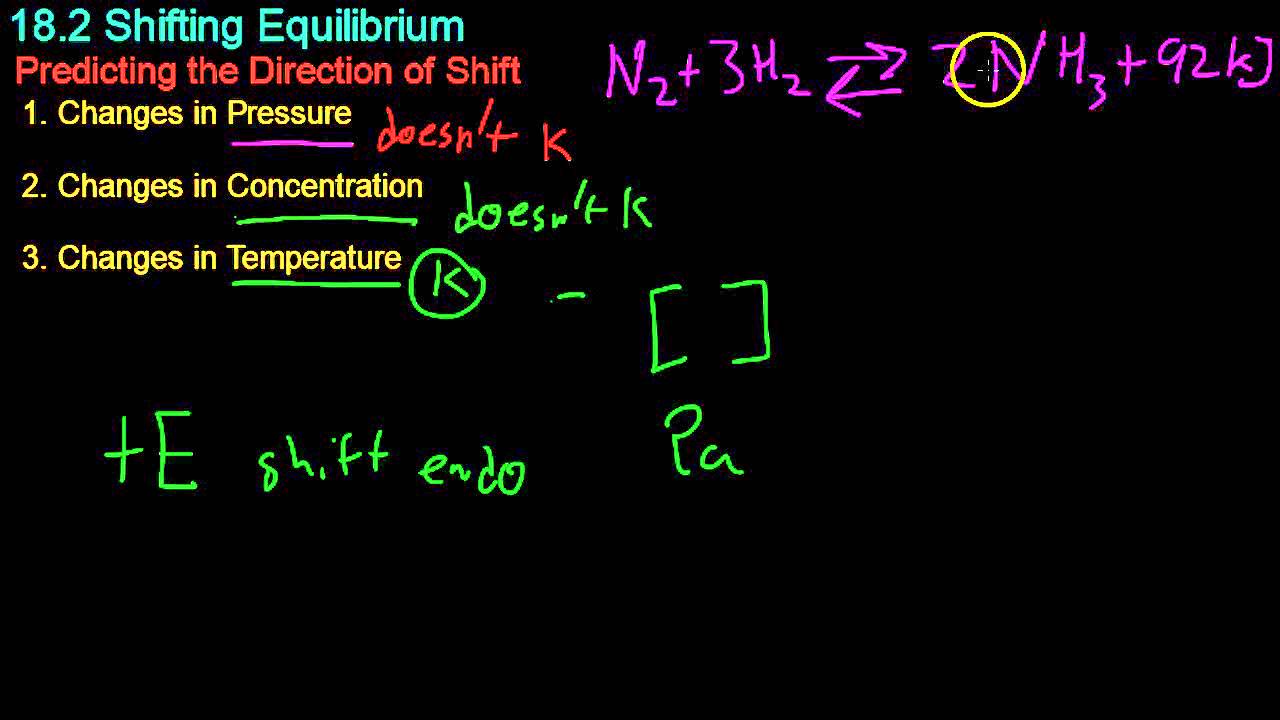
- 😀 Shifts in equilibrium can be caused by three main factors: changes in concentration, temperature, and pressure/volume.
- 😀 Increasing the concentration of a substance in a reaction shifts the equilibrium to decrease the concentration of that substance.
- 😀 Decreasing the concentration of a substance shifts the equilibrium to increase the concentration of that substance.
- 😀 For temperature changes, reactions involving heat are classified as exothermic (releasing heat) or endothermic (absorbing heat), and equilibrium shifts accordingly.
- 😀 If the temperature is increased, the equilibrium shifts towards the endothermic side to absorb the added heat.
- 😀 If the temperature is decreased, the equilibrium shifts towards the exothermic side to release heat.
- 😀 Pressure changes, particularly for reactions involving gases, will shift equilibrium to the side with fewer gas molecules.
- 😀 Reducing the volume or increasing pressure in a gaseous system shifts equilibrium towards the side with fewer moles of gas.
- 😀 Adding an inert gas does not affect the equilibrium position since inert gases do not participate in the reaction.
- 😀 Changes in pressure or volume do not affect equilibrium when the total number of moles of reactants and products is the same.
Q & A
What is Le Chatelier's principle and how does it relate to equilibrium shifts?
-Le Chatelier's principle states that if a system at equilibrium is disturbed by external factors, the system will shift to counteract that disturbance, reaching a new equilibrium. This principle applies to changes in concentration, temperature, pressure, and volume in a system at chemical equilibrium.
What are the three factors that can cause shifts in equilibrium?
-The three factors that can cause shifts in equilibrium are changes in the concentration of reactants or products, changes in temperature, and changes in pressure or volume.
How does changing the concentration of a substance affect the equilibrium of a reaction?
-If the concentration of a substance is increased, the equilibrium will shift to reduce the concentration of that substance by moving in the direction that consumes it. Conversely, if the concentration is decreased, the equilibrium shifts to produce more of the substance.
What happens when the concentration of KN03 is increased in a system at equilibrium?
-When the concentration of KN03 is increased in a system at equilibrium, the equilibrium shifts to the right, towards the production of more products, and the solution turns a deeper red.
How does temperature affect chemical equilibrium?
-Temperature changes affect equilibrium based on whether the reaction is exothermic or endothermic. If temperature is increased, the equilibrium shifts towards the endothermic direction, and if temperature is decreased, it shifts towards the exothermic direction.
What is the effect of raising the temperature in an endothermic reaction?
-Raising the temperature in an endothermic reaction shifts the equilibrium towards the products, as the system absorbs the added heat.
How does the change in pressure or volume affect gaseous equilibria?
-Changes in pressure or volume affect gaseous equilibria by shifting the equilibrium towards the side with fewer gas molecules. Increasing pressure or decreasing volume shifts the equilibrium to the side with fewer moles of gas, while decreasing pressure or increasing volume shifts it to the side with more gas molecules.
What happens when the pressure of a system involving gases is increased?
-Increasing the pressure of a gaseous system shifts the equilibrium to the side with fewer gas molecules, minimizing the impact of the pressure change.
How does adding an inert gas to a system in equilibrium affect the equilibrium?
-Adding an inert gas to a system at equilibrium does not affect the position of the equilibrium, as the inert gas does not react with any of the components in the system.
How does the value of Kc relate to the direction of equilibrium shifts?
-The value of the equilibrium constant, Kc, changes depending on the direction of the equilibrium shift. If the equilibrium shifts towards the products, Kc increases, and if it shifts towards the reactants, Kc decreases.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео

Deslocamento de Equilíbrio - Princípio de Le Chatelier

Faktor-Faktor yang Mempengaruhi Pergeseran Kesetimbangan dan Penerapannya dalam Industri

11 клас. Хімія. Необоротні та оборотні хімічні реакції. Хімічна рівновага. Принцип Ле Шательє
18.2 Shifting Equilibrium

Kesetimbangan Kimia | Faktor Yang Mempengaruhi Pergeseran Kesetimbangan

LeChatelier Principle: Change Pressure
5.0 / 5 (0 votes)
