S9Q2W8 | PERCENT COMPOSITION OF COMPOUNDS
Summary
TLDRThis lesson on percent composition teaches how to determine the percentage by mass of each element in a compound. Using examples like a three-in-one coffee sachet and the compound ethanol, the video explains how to calculate mass percent using the formula: (mass of element/mass of compound) × 100%. Key steps include finding the molar mass by summing atomic masses and applying the formula for each element. The video emphasizes rounding off to the nearest hundredths and checking that the total percent composition sums to 100%. Understanding these concepts is essential for laboratory work and analyzing chemical substances to determine empirical formulas.
Takeaways
- 😀 Percent composition refers to the percentage by mass of each element in a compound.
- 😀 You can calculate percent composition by dividing the mass of each element by the total mass of the compound and multiplying by 100.
- 😀 An example of percent composition calculation is in a 3-in-1 coffee sachet, with components of coffee, sugar, and milk.
- 😀 The mass percent of coffee in the 3-in-1 sachet is 28.57%, sugar is 37.14%, and milk is 34.29%.
- 😀 In any compound, the sum of the mass percents of its components must equal 100%.
- 😀 The formula for mass percent is: (mass of element / mass of compound) × 100.
- 😀 To calculate percent composition, you first need to know the molar mass of the compound and its individual elements.
- 😀 An example with ethanol (C₂H₆O) shows how to calculate the percent composition of carbon, hydrogen, and oxygen.
- 😀 The mass percent of carbon in ethanol is 52.14%, hydrogen is 13.14%, and oxygen is 34.7%.
- 😀 It is essential to round off results to the nearest hundredth to ensure accuracy in the percent composition.
- 😀 Percent composition is crucial in chemistry for tasks like preparing solutions and verifying the purity of substances.
Q & A
What is percent composition in chemistry?
-Percent composition refers to the percentage by mass of each element in a compound. It is calculated by dividing the mass of an element in the compound by the total mass of the compound and multiplying by 100.
How do you calculate the percent composition of an ingredient in a mixture, like instant coffee?
-To calculate the percent composition of an ingredient, divide the mass of that ingredient by the total mass of the mixture, then multiply the result by 100. For example, for coffee in a 35g sachet, 10g of coffee would give a percent composition of 28.57%.
Why is it important to round off the results when calculating percent composition?
-Rounding off ensures that the results are in a standardized format, making them easier to interpret and compare. It also helps to minimize minor calculation errors when dealing with significant figures.
What is the formula for mass percent composition?
-The formula for mass percent composition is: Mass percent = (Mass of element / Mass of compound) × 100.
In the example of ethanol, how is the molar mass calculated?
-The molar mass of ethanol (C2H6O) is calculated by adding the molar masses of its components: Carbon (12.01 g/mol × 2), Hydrogen (1.008 g/mol × 6), and Oxygen (16.0 g/mol × 1), resulting in a total molar mass of 46.07 g/mol.
What is the percent composition of carbon in ethanol?
-The percent composition of carbon in ethanol is calculated as (24.02 g / 46.07 g) × 100 = 52.14%.
How do you check if the percent compositions of a compound add up correctly?
-You can check the accuracy of the percent compositions by adding up the percentages of all the elements in the compound. They should total 100%, though small rounding deviations may cause minor differences.
Why is percent composition important in laboratory experiments?
-Percent composition is crucial in laboratory experiments for preparing solutions, determining the purity of substances, and analyzing chemical reactions. It helps to calculate precise amounts of substances and ensure the correctness of experimental outcomes.
What is the percent composition of hydrogen and oxygen in ethanol?
-The percent composition of hydrogen in ethanol is 13.14%, and the percent composition of oxygen is 34.7%.
What can affect the percent composition calculations in real experiments?
-In real experiments, factors like impurities in the compound, measurement inaccuracies, and rounding errors can affect the accuracy of percent composition calculations.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео

Percent Compositions of Compound| Grade 9 Science Quarter 2 Week 8 | DepEd MELC-based
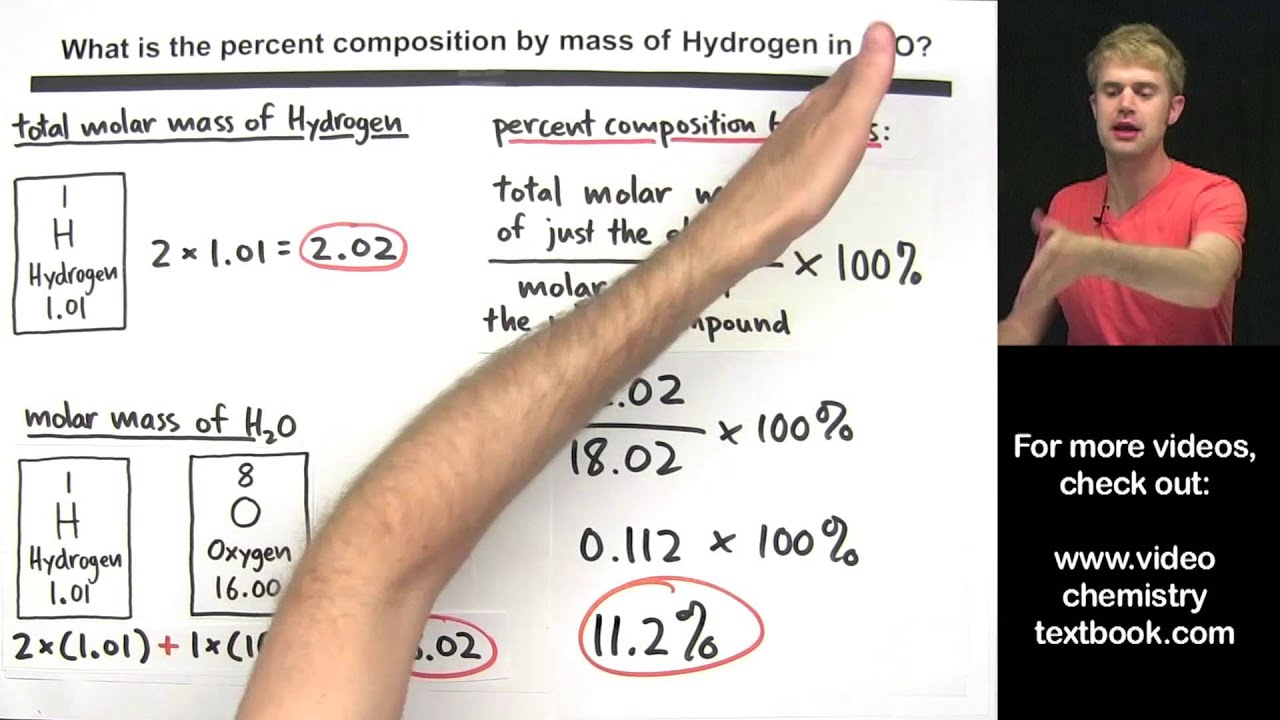
Percent Composition By Mass
How to Find the Percent Composition by Mass for Zn(OH)2 (Zinc hydroxide)

How to Find the Percent Composition by Mass for a Compound

Empirical Formula from Percent Composition

Stoikiometri (4) | Menentukan Kadar Unsur dalam Senyawa | Kimia Kelas 11
5.0 / 5 (0 votes)
