What are Isotopes?
Summary
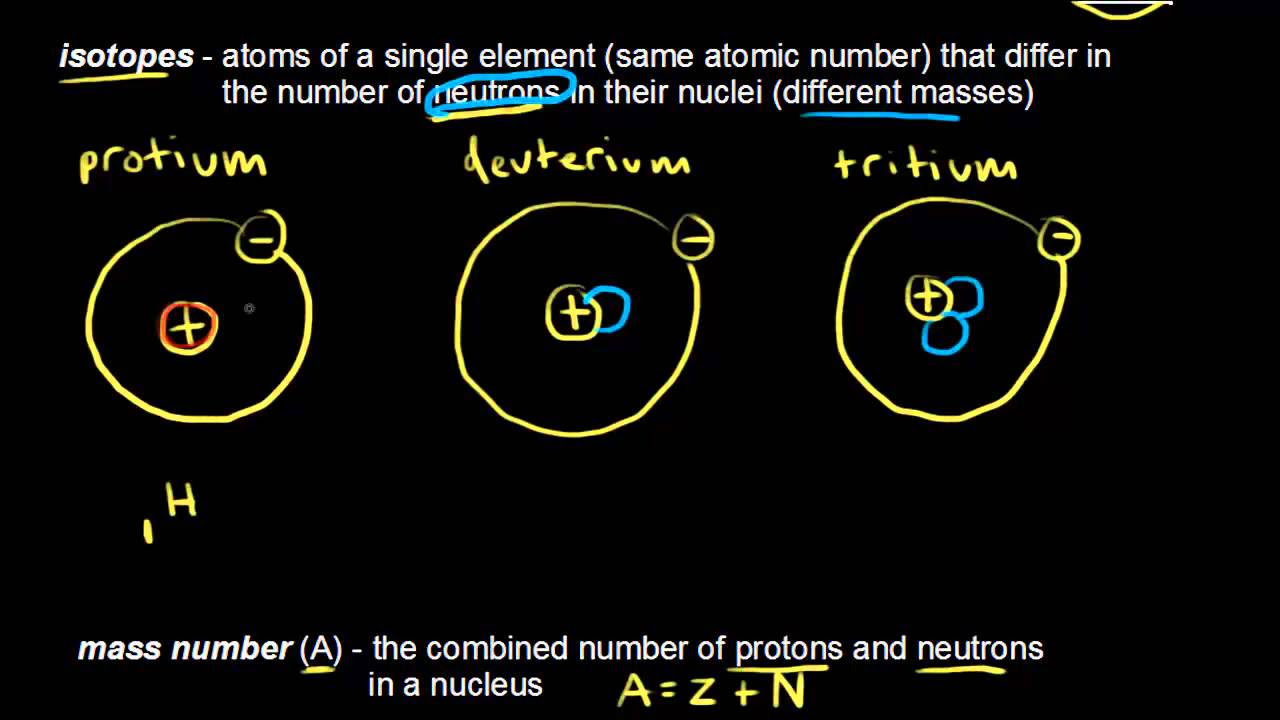
TLDRIsotopes are variations of the same element that differ in the number of neutrons while maintaining the same number of protons. Using the analogy of a fictional luxury car, the Lamona, with different models representing different isotopes, the video explains how carbon has three isotopes: carbon-12, carbon-13, and carbon-14. Each has six protons but varying neutrons. The transcript also discusses the importance of atomic and mass numbers and introduces isotopes of other elements, such as calcium and iron, highlighting the concept of isotopes in chemistry.
Takeaways
- 😀 Isotopes are different versions of the same element, differing in the number of neutrons.
- 🚗 An analogy of cars helps explain isotopes: different models can have the same basic shape but vary in features.
- 🍋 The example car, 'Lamona,' illustrates how isotopes maintain the same elemental identity despite differences.
- 🔬 Carbon has three main isotopes: Carbon-12, Carbon-13, and Carbon-14, each with six protons.
- 🔢 The atomic number, which is the number of protons, determines the element type.
- ⚖️ The mass number is the sum of protons and neutrons, differentiating isotopes of the same element.
- ✍️ Isotope notation displays an element's atomic number and mass number (e.g., Carbon-12 as _{6}^{12}C).
- 🧪 Many elements have isotopes, including calcium and iron, each with their specific atomic and mass numbers.
- 📊 Isotopes are common in chemistry, playing a crucial role in various applications and research.
- 🔍 Understanding isotopes helps in fields like medicine, archaeology, and environmental science.
Q & A
What are isotopes?
-Isotopes are different versions of the same element that have the same number of protons but different numbers of neutrons.
How does the analogy of the Lamona car help explain isotopes?
-The Lamona car analogy illustrates that just like different models of the same car can have various features while retaining the same design, isotopes of an element differ by their neutron count while having the same number of protons.
What are the three isotopes of carbon mentioned in the transcript?
-The three isotopes of carbon are Carbon-12 (6 protons, 6 neutrons), Carbon-13 (6 protons, 7 neutrons), and Carbon-14 (6 protons, 8 neutrons).
What is the significance of the atomic number?
-The atomic number is the number of protons in the nucleus of an atom, which defines the element. For carbon, the atomic number is 6.
How is the mass number calculated?
-The mass number is calculated by adding the number of protons and neutrons in the nucleus.
What is isotope notation?
-Isotope notation is a way of representing isotopes, where the atomic number is written at the bottom left and the mass number at the top left of the chemical symbol. For example, Carbon-12 is represented as 12C with the atomic number 6 below it.
Can other elements also have isotopes?
-Yes, almost all elements have multiple isotopes. For example, calcium has isotopes such as Calcium-40, Calcium-42, and others, all having the same atomic number but different mass numbers.
What is the atomic number of calcium, and how many isotopes are mentioned?
-Calcium has an atomic number of 20, and the transcript mentions several isotopes with varying mass numbers, including Calcium-40, Calcium-42, Calcium-43, Calcium-44, Calcium-46, and Calcium-48.
What is the atomic number of iron, and how does it relate to its isotopes?
-Iron has an atomic number of 26, meaning it has 26 protons in its nucleus. Its isotopes differ by the number of neutrons but maintain the same atomic number.
What is the key takeaway regarding isotopes?
-The key takeaway is that isotopes are variations of an element defined by their proton count, with differences in neutron numbers not changing the element's identity.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео
5.0 / 5 (0 votes)