Metallic Bonding and the Electron Sea Model, Electrical Conductivity - Basic Introduction
Summary
TLDRThe video explains metallic bonding and why metals conduct electricity. It describes how valence electrons in metals are delocalized, allowing them to move freely among metal cations, unlike core electrons which are localized. This electron mobility is what enables metals to conduct electricity. The video also highlights how applying a voltage across a metal creates an electric field that directs the flow of electrons. Additionally, it covers the physical properties of metals, such as malleability, ductility, thermal conductivity, and luster, and notes how their melting points vary widely depending on the type of metal.
Takeaways
- 🔗 Metallic bonds are a form of covalent bonding where metal atoms share valence electrons.
- 🧲 The valence electrons in metals are free to move, which makes them 'delocalized' and allows them to conduct electricity.
- ⚡ Metals conduct electricity due to the free movement of delocalized electrons within the metal structure.
- 🔋 The application of an electric field (e.g., from a battery) aligns the movement of electrons in one direction, enabling current flow.
- 🔨 Metals are malleable, meaning they can be hammered into thin sheets without breaking.
- 🔧 Metals are ductile, meaning they can be drawn into thin wires, which is why they are used in electrical wiring.
- 🔥 Metals are excellent thermal conductors because the free-moving electrons allow for quick transfer of heat across the material.
- ✨ Metals exhibit luster, giving them a shiny appearance, which is a common physical characteristic.
- 🌡️ Metals have varying melting points, from low (like mercury and gallium) to extremely high (like tungsten).
- 🚿 Metals can conduct both heat and electricity because of their free-moving valence electrons, which are not bound to individual atoms.
Q & A
What is metallic bonding?
-Metallic bonding is a type of chemical bond where valence electrons are shared between many metal cations. These electrons are free to move and are not bound to any specific atom, creating a 'sea of electrons.'
How are metallic bonds different from typical covalent bonds?
-While metallic bonds involve the sharing of electrons like covalent bonds, the difference is that in metallic bonds, the electrons are shared among thousands of atoms, rather than just two atoms as in covalent bonds found in molecules.
What is meant by 'delocalized' electrons in metals?
-Delocalized electrons in metals are valence electrons that are not attached to any particular atom. They are free to move throughout the metal, allowing metals to conduct electricity.
Why are core electrons not delocalized in metals?
-Core electrons are localized, meaning they are bound to a specific atom and cannot move freely like valence electrons. This is why they do not contribute to the conductivity of metals.
Why can metals conduct electricity?
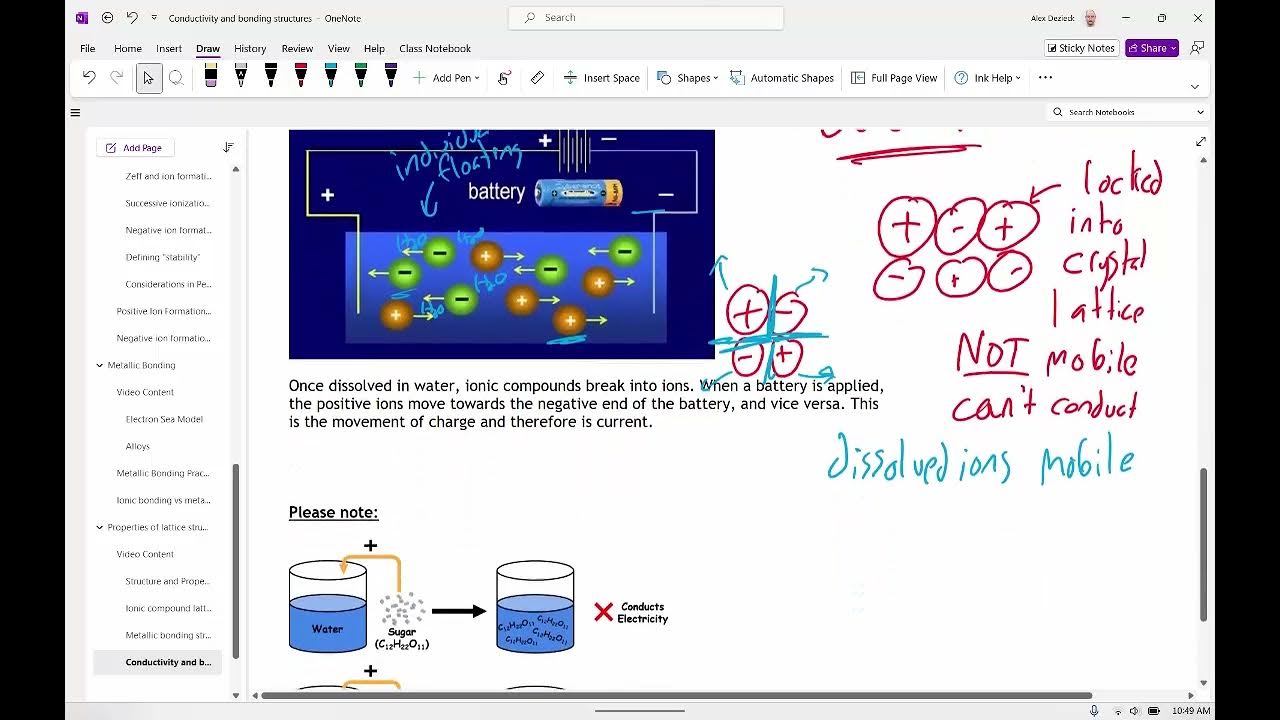
-Metals can conduct electricity because their valence electrons are free to move. When an electric field is applied, these electrons flow, creating an electric current.
What is the role of a battery in a metal wire circuit?
-A battery creates a voltage that sets up an electric field across a metal wire. This field causes the already present electrons in the wire to flow in one direction, creating an electric current.
What does 'malleable' mean in the context of metals?
-Malleable means that metals can be hammered or pressed into thin sheets without breaking. This is due to the flexibility of the metallic bonds.
What does 'ductile' mean when describing metals?
-Ductile refers to the ability of metals to be stretched or drawn into thin wires. This is another property of metals resulting from their flexible bonding.
Why are metals good conductors of heat?
-Metals are good conductors of heat because their delocalized electrons can transfer thermal energy quickly from one part of the metal to another.
How do the melting points of metals vary?
-The melting points of metals vary widely. For example, zinc has a relatively low melting point of 420°C, while tungsten has a very high melting point of about 3400°C. Some metals like mercury are liquid at room temperature.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео

What Are Metallic Bonds | Properties of Matter | Chemistry | FuseSchool
Conductivity and Reflectivity

IKATAN LOGAM ADALAH

LIGAÇÕES QUÍMICAS | REGRA DO OCTETO | IÔNICA, COVALENTE E METÁLICA | REVISÃO

How Do Atoms Bond - Part 2 | Properties of Matter | Chemistry | FuseSchool

Ikatan Logam: Pengertian, Sifat, dan Contoh dalam Kehidupan Sehari-hari
5.0 / 5 (0 votes)
