Law of Conservation of Mass Example
Summary
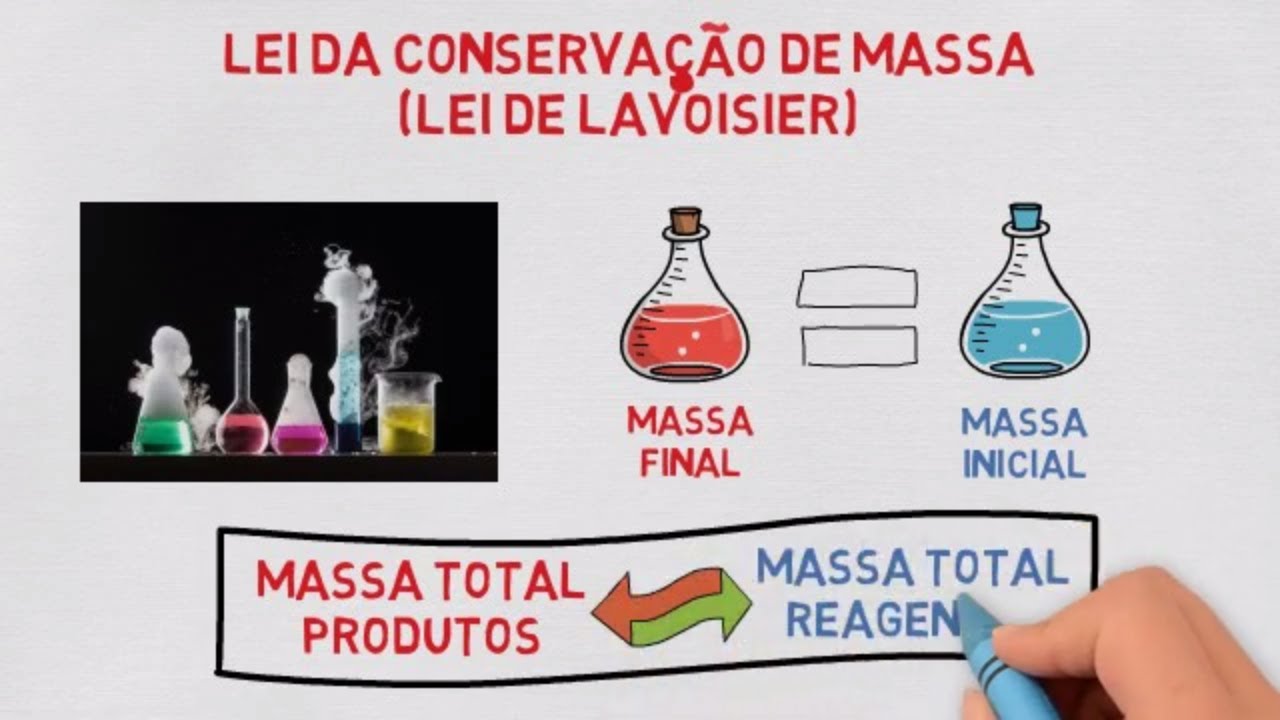
TLDRThe video explains the law of conservation of mass, which states that mass is neither created nor destroyed in a chemical reaction. This principle was established in 1789 by French chemist Antoine Lavoisier. A demonstration using baking soda and vinegar shows that after a reaction occurs, the mass of the products remains the same as the mass of the reactants. The elements involved are simply rearranged, not lost or gained. The video highlights how this fundamental law applies in everyday chemical reactions.
Takeaways
- 🔬 The mass of reactants remains the same before and after a chemical reaction.
- 📜 The law of conservation of mass states that mass cannot be created or destroyed in chemical reactions.
- 🧑🔬 This law was formulated by a French chemist in 1789.
- ⚖️ According to this law, the mass of the products must equal the mass of the reactants in a chemical reaction.
- 📏 In the example, the total mass of the reactants (113 grams) remains the same after the reaction.
- 💥 Mixing baking soda and vinegar demonstrates the law of conservation of mass.
- 🌬 Even though gas is produced in the reaction, the total mass remains unchanged.
- 🔄 The mass is not destroyed or created; it is simply rearranged.
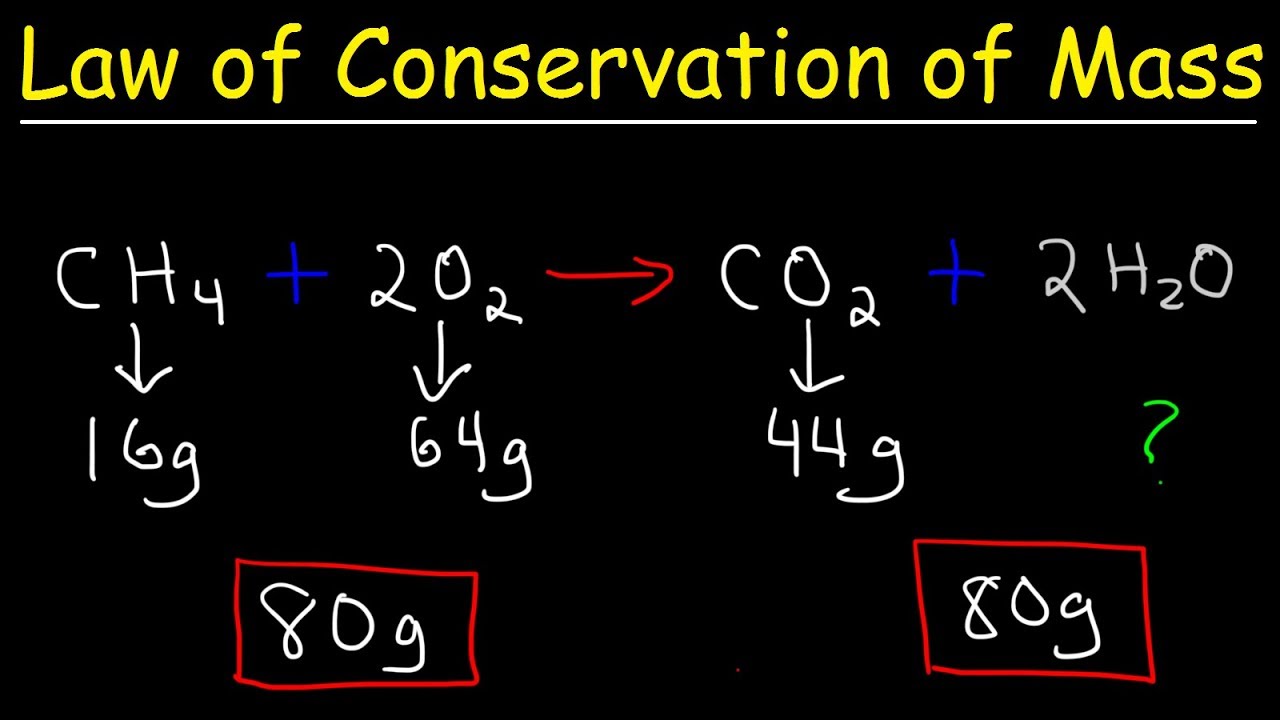
- 🧪 The balanced equation of the reaction shows that the elements are equal on both sides.
- 📚 The law of conservation of mass applies in everyday life, and understanding it can be further explored through educational resources.
Q & A
What does the law of conservation of mass state?
-The law of conservation of mass states that mass is neither created nor destroyed by chemical reactions.
Who formulated the law of conservation of mass, and when?
-The law of conservation of mass was formulated in 1789 by a French chemist.
How does the law of conservation of mass apply to chemical reactions?
-According to the law of conservation of mass, the mass of the products in a chemical reaction must equal the mass of the reactants.
What happens to the mass of reactants and products during a chemical reaction?
-The mass of the reactants and products remains the same before and after a chemical reaction; the mass is not created or destroyed, just rearranged.
What example is used in the script to demonstrate the law of conservation of mass?
-The reaction between baking soda and vinegar is used as an example, where the mass before and after the reaction remains 113 grams.
Why does the mass remain constant during the baking soda and vinegar reaction?
-The mass remains constant because the elements in the reactants are rearranged but not destroyed or created, thus maintaining the same total mass.
What is the significance of a balanced chemical equation in relation to the law of conservation of mass?
-A balanced chemical equation shows that the elements on both sides of the equation are equal, supporting the law of conservation of mass by demonstrating that the mass is conserved.
How is the production of gas accounted for in the mass of products during a reaction?
-Even though gas is produced during a reaction, the total mass of the system remains the same because the mass of the gas is part of the overall mass.
What happens to the elements in a chemical reaction according to the law of conservation of mass?
-The elements are rearranged during the reaction, but their total mass stays the same as before.
What practical applications does the law of conservation of mass have in everyday life?
-The law of conservation of mass can be observed in everyday chemical reactions, like baking soda and vinegar, showing that mass is conserved in all types of reactions.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео
5.0 / 5 (0 votes)