Nuclear Chemistry Part 2 - Fusion and Fission: Crash Course Chemistry #39
Summary
TLDRThis Crash Course Chemistry episode delves into nuclear chemistry, explaining the pursuit of stability in atomic nuclei through binding energy. It introduces Einstein's E=mc² to calculate energy from mass defect, highlighting the significance of nuclear energy in both fission and fusion reactions. The video contrasts the controlled use of fission in power plants with its uncontrolled potential in nuclear weapons, while discussing the challenges and applications of fusion, including the sun's energy production. It concludes by encouraging further exploration in nuclear chemistry to harness its potential safely and efficiently.
Takeaways
- 🔬 Chemistry, like life, seeks stability, and in nuclear chemistry, this stability is about maintaining the nucleus' integrity.
- ⚛️ Radioactive decay is a process where atomic nuclei shed particles to achieve greater stability.
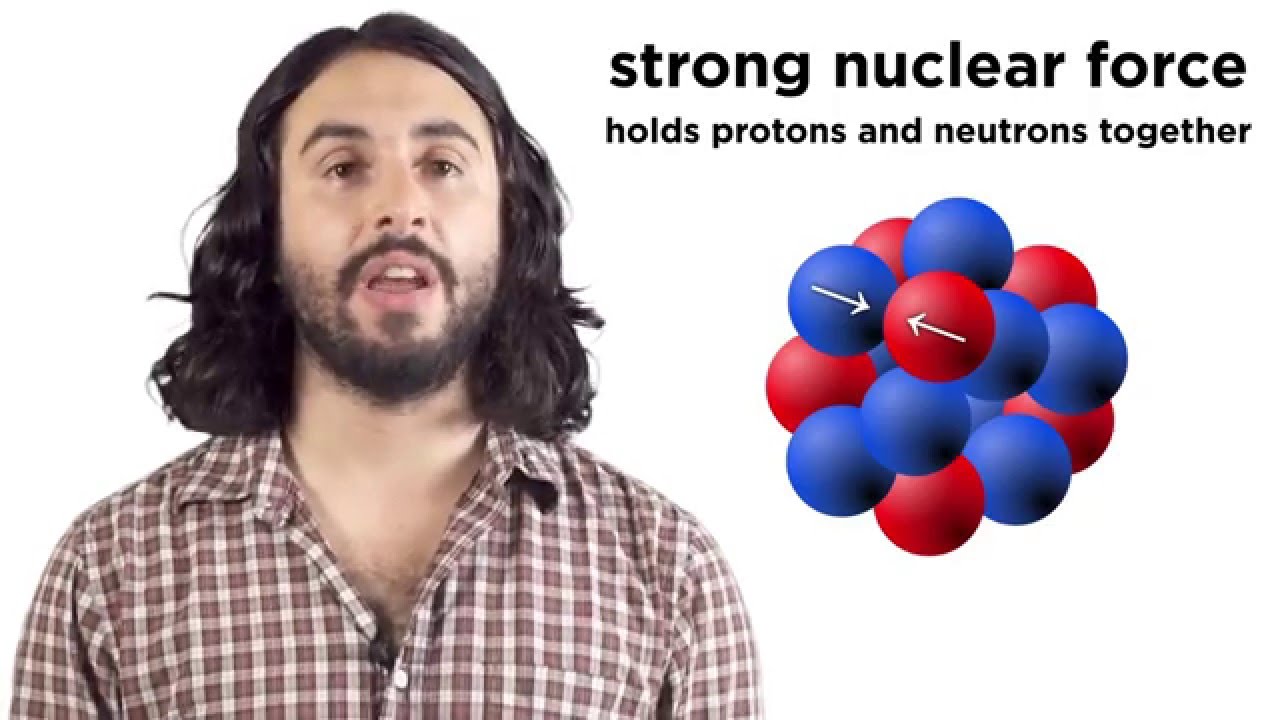
- 🧲 Binding energy is the force that holds protons and neutrons together in an atom's nucleus, and it's a fundamental concept in nuclear chemistry.
- 🌟 Einstein's E=mc² formula is used to calculate the binding energy of an atom, demonstrating that mass can be converted into energy.
- 🔍 The mass of an atom's nucleus is less than the sum of its individual nucleons, and this 'missing mass' is the mass defect, which is equivalent to energy.
- ⚡ The energy released in nuclear reactions, such as fission and fusion, is immense, making nuclear energy a potent source of power.
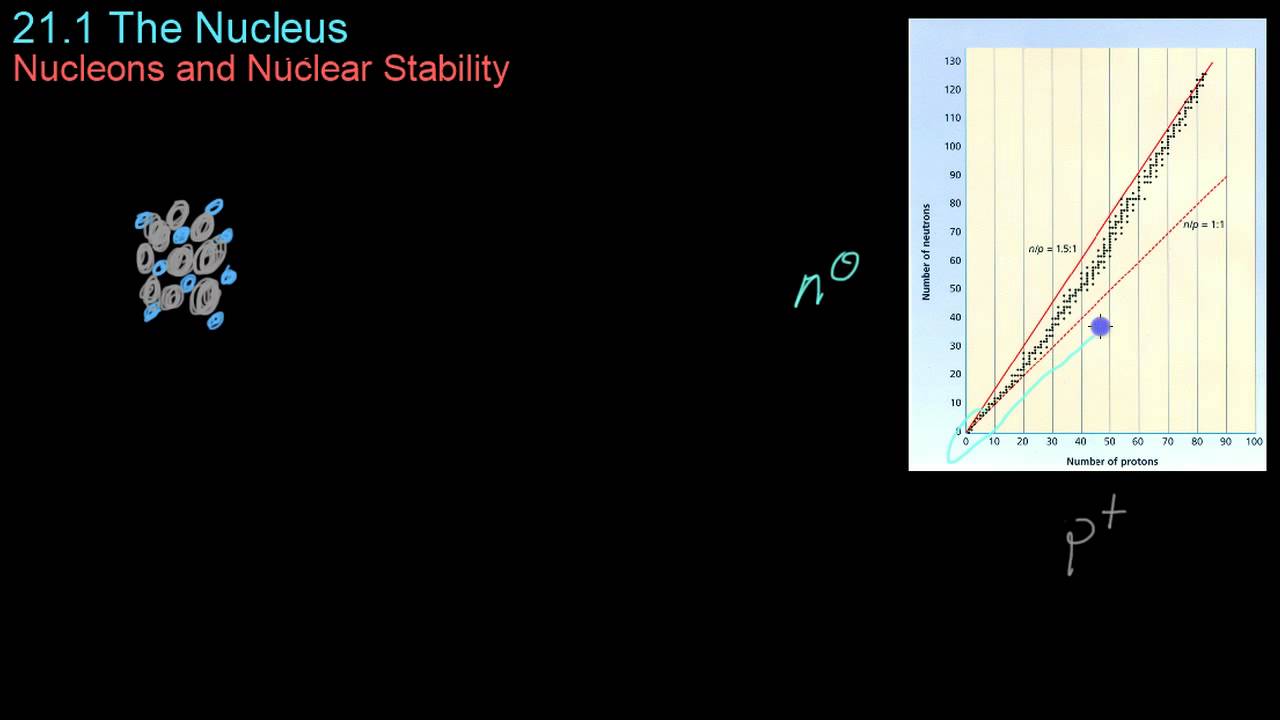
- 📉 Elements with higher binding energies, like iron-56, are very stable and less likely to undergo nuclear reactions.
- 💥 Fission is a nuclear reaction where a large nucleus splits into smaller ones, releasing energy, while fusion is the combination of light nuclei to form a heavier nucleus.
- ⚙️ Nuclear power plants use controlled fission reactions to generate electricity, but they must manage the risk of uncontrolled chain reactions.
- ♻️ Nuclear reactions produce radioactive waste with varying half-lives, posing long-term storage and disposal challenges.
- 🌞 Fusion reactions, like those in the sun, release vast amounts of energy, but controlling them for practical use on Earth remains a significant challenge.
Q & A
What is the concept of stability in nuclear chemistry?
-In nuclear chemistry, stability refers to the ability of an atomic nucleus to remain intact without breaking apart. It is associated with the balance of forces that hold the nucleus together.
What is binding energy and why is it significant?
-Binding energy is the amount of energy that holds protons and neutrons together in an atomic nucleus. It is significant because it is a fundamental principle of nuclear chemistry and is directly related to the stability of the nucleus. The binding energy is released or absorbed during nuclear reactions, such as fission or fusion.
How is the binding energy of an atom calculated?
-The binding energy of an atom is calculated using Einstein's mass-energy equivalence formula, E=mc^2, where 'm' is the mass defect (the difference between the mass of individual nucleons and the mass of the nucleus), 'c' is the speed of light, and 'E' is the binding energy.
What is the mass defect and how does it relate to energy?
-The mass defect is the difference in mass between the individual nucleons (protons and neutrons) of an atom and the mass of the nucleus as a whole. This 'missing mass' is converted into energy, which is the binding energy that holds the nucleus together.
What are the two general types of nuclear reactions that release energy?
-The two general types of nuclear reactions that release energy are fission, where a large nucleus splits into two lighter ones, and fusion, where two light nuclei join together to form a heavier one.
How does the binding energy curve relate to the stability of elements?
-The binding energy curve shows that elements with higher binding energies per nucleon, such as iron-56, are more stable and less likely to undergo nuclear reactions. Elements with lower binding energies per nucleon are less stable and more likely to undergo fission or fusion reactions to achieve greater stability.
What is the role of uranium-235 in nuclear reactions?
-Uranium-235 is a common fuel for nuclear fission reactions. It can be triggered to split into smaller atoms by absorbing neutrons, releasing energy, more neutrons, and other byproducts.
How do nuclear power plants control the fission chain reaction?
-Nuclear power plants control the fission chain reaction by using control rods made of materials that absorb neutrons. These rods can be inserted or removed to regulate the rate of the reaction, thus controlling the release of energy.
What are the challenges associated with the waste products of nuclear fission?
-The waste products of nuclear fission are radioactive and can remain hazardous for very long periods, ranging from a few years to millions of years. These radioactive materials need to be stored safely to prevent environmental contamination.
How does fusion differ from fission in terms of energy release and control?
-Fusion reactions release even more energy than fission due to the larger energy changes involved in combining light nuclei. However, fusion reactions are harder to control because they require extremely high temperatures and pressures to overcome the electrostatic repulsion between nuclei.
What are the applications and limitations of nuclear fusion on Earth?
-On Earth, controlled nuclear fusion has not yet been achieved for practical energy generation due to the technical challenges of reaching and maintaining the necessary conditions. However, uncontrolled fusion reactions are used in thermonuclear weapons.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тариф5.0 / 5 (0 votes)