OCR A 2.1.1 Atomic Structure and Isotopes REVISION
Summary
TLDRIn this educational video, Chris Harris from Allert Tutors explores atomic structure and isotopes as per the OCR specification. Harris explains the atom's components, including protons, neutrons, and electrons, and their charges and relative masses. He delves into ions, isotopes, and their differences, using oxygen as an example. The video also covers the historical development of atomic models, from Dalton's spherical atoms to Rutherford's nuclear model and Bohr's energy shells. Harris teaches how to calculate relative atomic mass using mass spectra, a method for measuring isotope masses, and identifies elements like chlorine and germanium through this process. The video is a valuable resource for students revising for exams, with supplementary PowerPoints available for purchase.
Takeaways
- 🔬 The video provides a revision overview of atomic structure and isotopes for the OCR specification.
- 🌐 The presenter, Chris Harris from allertutors.com, offers PowerPoints for purchase to aid in revision and note-taking.
- 💡 Atoms consist of a nucleus with protons and neutrons, and electrons orbiting in shells, with the nucleus being very small relative to the atom's size.
- ⚛️ Protons have a relative charge of +1 and mass of 1, neutrons have no charge and a relative mass of 1, and electrons have a charge of -1 and a very small relative mass.
- 📊 The periodic table lists elements with a mass number (larger number) indicating the sum of protons and neutrons, and an atomic or proton number (smaller number) indicating the number of protons.
- 🔋 The number of protons in an atom equals the number of electrons, and the number of neutrons can be calculated by subtracting the atomic number from the mass number.
- ⚡ Ions are atoms that have lost or gained electrons, resulting in a different number of electrons and protons, unlike neutral atoms.
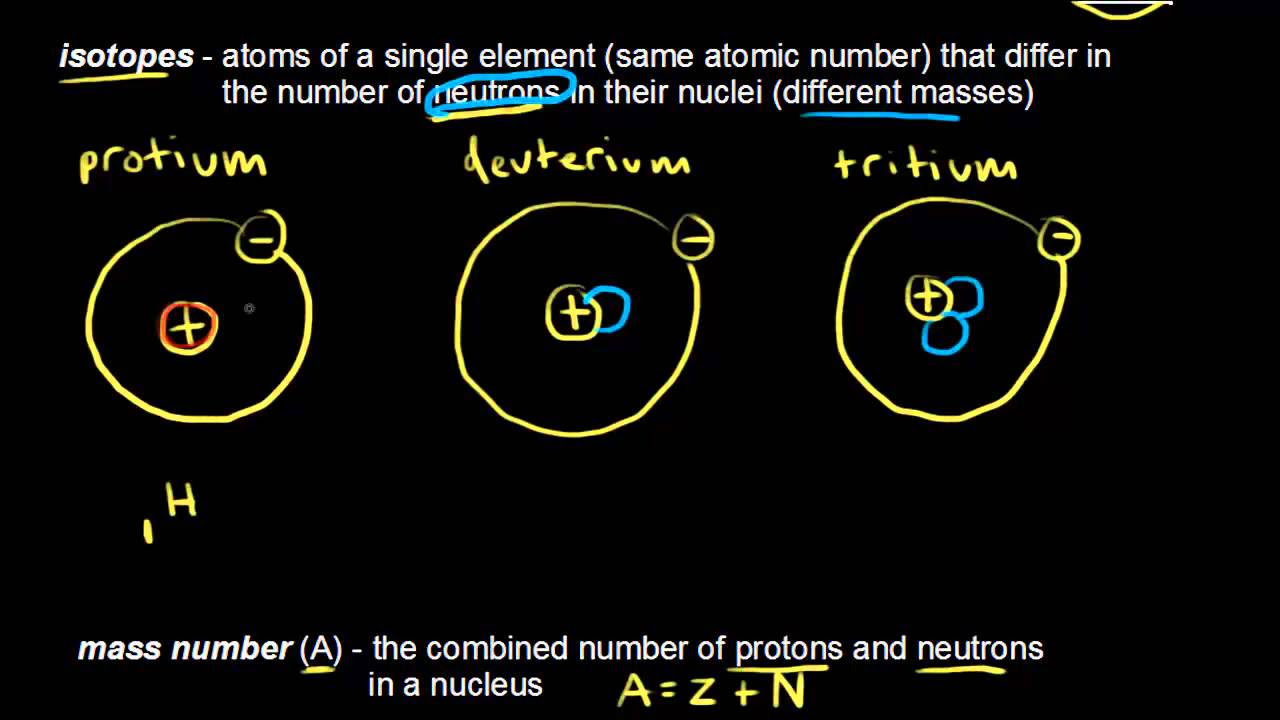
- 🌐 Isotopes are variants of an element with the same number of protons but different numbers of neutrons, leading to different masses.
- 📈 The history of atomic models includes significant contributions from John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr, each refining the understanding of atomic structure.
- 🧬 Mass spectrometry is a technique used to measure the mass of isotopes in an element, and the relative atomic mass of an element can be calculated from the mass spectrum data.
Q & A
What is the main topic of the video presented by Chris Harris?
-The main topic of the video is the atomic structure and isotopes, specifically tailored for the OCR specification.
How can viewers obtain the PowerPoints used in the video?
-Viewers can purchase the PowerPoints by clicking on the link provided in the description box below the video.
What are the three main components of an atom as described in the video?
-The three main components of an atom are protons, neutrons, and electrons.
What is the relative charge and mass of a proton as mentioned in the video?
-A proton has a relative charge of plus one and a relative mass of one.
How does the video explain the difference between the number of protons and neutrons in determining the mass number of an element?
-The mass number of an element is determined by the sum of the number of protons and neutrons in the nucleus.
What is an ion according to the video?
-An ion is an atom that has lost or gained electrons, resulting in a different number of electrons and protons.
What is the key difference between isotopes as explained in the video?
-Isotopes are variants of an element with the same number of protons but different numbers of neutrons.
How does the video describe the historical development of the atomic model from John Dalton to Neils Bohr?
-The video outlines the progression from Dalton's solid sphere model, to Thomson's plum pudding model, Rutherford's discovery of the nucleus, and finally to Bohr's model with fixed energy shells.
What is the significance of the gold leaf experiment in the context of the video?
-The gold leaf experiment, conducted by Rutherford, provided evidence for the existence of a small, positively charged nucleus within the atom by observing the deflection of alpha particles.
How can the relative atomic mass of an element be calculated from its isotopes, as per the video?
-The relative atomic mass can be calculated by multiplying the mass of each isotope by its percentage abundance and summing these products, then dividing by the total percentage abundance.
What is the purpose of Mass Spectra as explained in the video?
-Mass Spectra is used to measure the mass of isotopes in an element by determining the mass-to-charge ratio of the ions produced when electrons are removed from the atoms.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео
5.0 / 5 (0 votes)