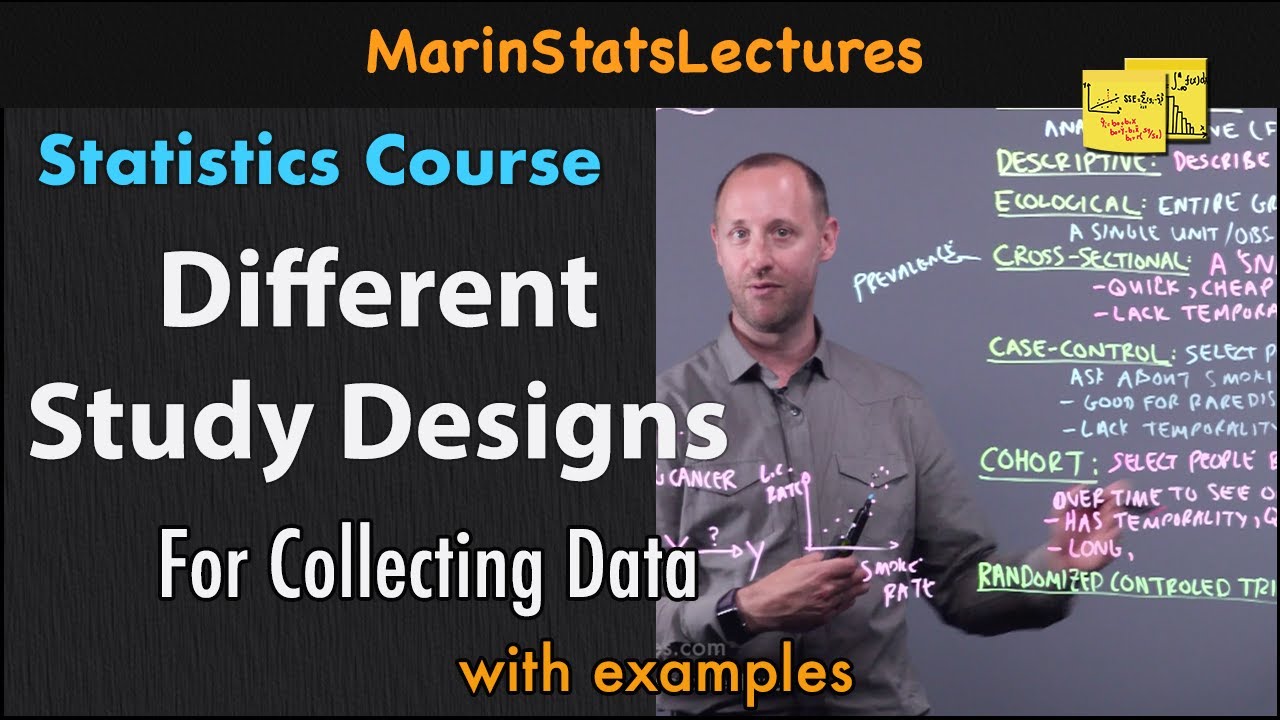
Case-control study explained
Summary
TLDRThis video script delves into the case-control study design, a cost-effective and straightforward observational method in clinical research. It explains how researchers compare exposures between a group with a specific outcome, like cancer, and a control group without it. The script highlights the study's retrospective nature and its limitations, such as recall and selection bias. It also references the influential work of Richard Doll and Bradford Hill, which established the link between smoking and lung cancer, emphasizing the importance of further studies to validate findings.
Takeaways
- 🔍 The case-control study is a common observational study design in clinical research, known for being cost-effective and easy to perform.
- 📊 Researchers use case-control studies to determine if an exposure is associated with a specific outcome.
- 🧑🔬 The study involves two groups: 'cases' with the outcome of interest, and 'controls' without the outcome, ideally matched to be similar to the cases.
- ⏳ Case-control studies are retrospective, meaning they look back in time to investigate exposures that may have led to the outcome.
- 🚬 An example given is evaluating the smoking habits of individuals with lung cancer (cases) compared to those without (controls).
- 🔑 The study's aim is to find a correlation, not causation, which requires further research for validation.
- 💡 Limitations include recall bias, where affected individuals may remember past events differently, and selection bias, affecting who enrolls in the study.
- 📚 The script references a historical case-control study by Richard Doll and Bradford Hill, which established the link between tobacco and lung cancer.
- 🔄 The need for meta-analysis to confirm the results of case-control studies is highlighted, as they are not conclusive on their own.
- 🚭 The script concludes with a public health message, emphasizing the known risks of smoking and encouraging viewers to quit.
- 👍 The video encourages viewers to like and subscribe for more content, indicating a call to action for viewer engagement.
Q & A
What is a case-control study in clinical research?
-A case-control study is an observational study design used in clinical research to determine if an exposure is associated with an outcome. It is relatively inexpensive and easy to design and perform.
What are the two groups observed in a case-control study?
-The two groups observed in a case-control study are 'cases', a group known to have the outcome, and 'controls', a group known to be free of the outcome.
How are cases and controls ideally matched in a case-control study?
-Ideally, controls should be as similar as possible to the cases to ensure that the comparison is valid and that any differences in outcomes can be attributed to the exposure of interest.
Why are case-control studies considered retrospective?
-Case-control studies are considered retrospective because researchers go back in time to investigate exposures that may have led to the outcome.
What is an example of how a case-control study might quantify exposure?
-An example of quantifying exposure in a case-control study could be evaluating the quantity of people in both the case and control groups who were smoking years prior to the outcome, such as the development of cancer.
What is the significance of the correlation found in a case-control study?
-The correlation found in a case-control study suggests a potential association between exposure and outcome, but it does not imply causation and requires further investigation.
What is recall bias and how does it affect case-control studies?
-Recall bias refers to the tendency of people affected by a certain condition to remember events in their past differently, which can affect the accuracy of the information collected in case-control studies.
What is selection bias and how can it occur in a case-control study?
-Selection bias occurs when some individuals are more likely to enroll in a case-control study than others, potentially skewing the results if, for example, certain groups are over- or under-represented.
Why might additional studies and a meta-analysis be required after a case-control study?
-Additional studies and a meta-analysis are required to validate and strengthen the results of a case-control study, ensuring that the findings are robust and not due to chance or bias.
Who were Richard Doll and Bradford Hill, and what is their contribution to case-control studies?
-Richard Doll and Bradford Hill are well-known for their case-control study that established a strong association between tobacco consumption and lung cancer, which was published in the 1950s.
How has the understanding of the link between smoking and lung cancer evolved since the studies by Doll and Hill?
-Since the studies by Doll and Hill, the link between smoking and lung cancer has become widely accepted and validated by subsequent research, including prospective cohort studies.
What is the final message conveyed in the video about smoking and lung cancer?
-The final message of the video is a public health reminder that smoking causes lung cancer and encourages viewers to stop smoking for their health.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示
Study Designs (Cross-sectional, Case-control, Cohort) | Statistics Tutorial | MarinStatsLectures

Introduction to Clinical Study Design: Introduction to Observational & Interventional Studies Part 2

Dasar Epidemiologi - Desain Penelitian Crossectional

Introdução aos Estudos Epidemiológicos - Resumo - Epidemiologia

Observational Study vs Experiment

Prompt Engineering = BS? (Must Watch)
5.0 / 5 (0 votes)
