Le Chatelier's Principle
Summary
TLDRIn this educational video, Professor Dave explores Le Chatelier's principle, illustrating how systems at equilibrium adjust to various stresses such as changes in concentration, temperature, and pressure. He explains that adding or removing reactants or products will shift the equilibrium, and how exothermic and endothermic reactions respond to temperature changes. The video also covers the impact of volume and pressure on gaseous equilibria, using Boyle's law to demonstrate how equilibrium shifts to accommodate increased or decreased pressure.
Takeaways
- 🔄 Le Chatelier's principle states that a change in a system at equilibrium will shift to counteract the change and restore balance.
- 🧪 Adding or removing reactants or products can unbalance an equilibrium, causing the forward or reverse reaction to speed up to restore it.
- 🔄 If more reactants are added, the equilibrium shifts to the right to consume them and produce more products.
- 🔄 Conversely, adding more products shifts the equilibrium to the left, producing more reactants.
- 🌡 Changes in temperature affect equilibrium based on whether the reaction is exothermic (releases heat) or endothermic (absorbs heat).
- ♨️ An exothermic reaction, indicated by a negative delta H, will shift towards the reactants when heated to reduce excess energy.
- ❄️ Cooling an exothermic reaction shifts the equilibrium towards products, as the system absorbs heat.
- 🌡 For endothermic reactions, heating will shift the equilibrium towards products (absorbing heat), and cooling will shift it towards reactants.
- 💥 Temperature changes are treated as changes in the 'concentration' of heat energy in the system.
- 🌌 Changes in volume or pressure, especially with gaseous reactions, can also shift equilibrium according to the number of moles of gas particles involved.
- 🎈 Decreasing the volume of a gaseous system increases pressure, causing the equilibrium to shift towards the side with fewer gas particles to reduce pressure.
- 📦 Increasing the volume decreases pressure, and the equilibrium shifts towards the side with more gas particles to increase pressure.
- 📚 Understanding Le Chatelier's principle helps predict how a system at equilibrium will respond to various stresses.
Q & A
What is Le Chatelier's principle?
-Le Chatelier's principle states that if a system at equilibrium is subjected to a change in conditions, the system will adjust to counteract the change and restore a new equilibrium.
How does adding a reactant affect an equilibrium system?
-Adding a reactant to an equilibrium system will cause the forward reaction to speed up, using up the additional reactants and producing more products to restore equilibrium, thus shifting the equilibrium to the right.
What happens if more products are added to an equilibrium system?
-Adding more products to an equilibrium system will cause the system to shift to the left, favoring the reverse reaction to produce more reactants and restore balance.
How does the removal of a component affect an equilibrium system?
-Selective removal of a component from an equilibrium system will cause the system to shift to produce more of that species, in an attempt to restore the balance that was disrupted.
What is the significance of delta H in determining the direction of an equilibrium shift due to temperature changes?
-Delta H, the change in enthalpy, indicates whether a reaction is exothermic (releases energy, delta H is negative) or endothermic (absorbs energy, delta H is positive). This dictates whether increasing or decreasing the temperature will shift the equilibrium to the left or right.
How does an exothermic reaction respond to an increase in temperature?
-For an exothermic reaction (negative delta H), increasing the temperature will shift the equilibrium to the left, favoring the endothermic reverse reaction to absorb the excess heat and relieve the stress.
What is the effect of cooling down an endothermic reaction on its equilibrium?
-Cooling down an endothermic reaction (positive delta H) will shift the equilibrium to the right, favoring the forward reaction to release energy and counteract the temperature decrease.
How does changing the volume of a container with gases affect the equilibrium involving gases?
-Decreasing the volume of a container with gases increases the pressure, causing the equilibrium to shift towards the side with fewer gas particles to reduce the pressure. Increasing the volume decreases the pressure, shifting the equilibrium towards the side with more gas particles.
What is Boyle's law and how does it relate to pressure changes in an equilibrium system?
-Boyle's law states that the pressure of a gas is inversely proportional to its volume at constant temperature. In an equilibrium system involving gases, a decrease in volume will increase the pressure, and vice versa, which can affect the direction of the equilibrium shift.
Can you provide an example of an equilibrium involving gases where a change in volume affects the equilibrium position?
-An example is the equilibrium between a diatomic molecule (like O2) and two monoatomic species (like 2O). If the volume is decreased, the equilibrium will shift to the left, favoring the formation of fewer gas particles (atoms) to reduce pressure.
What can we do to check the comprehension of the concepts discussed in the script?
-To check comprehension, one can review the script, engage in discussions, or answer questions related to Le Chatelier's principle, the effects of concentration, temperature, and pressure changes on equilibrium systems.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示
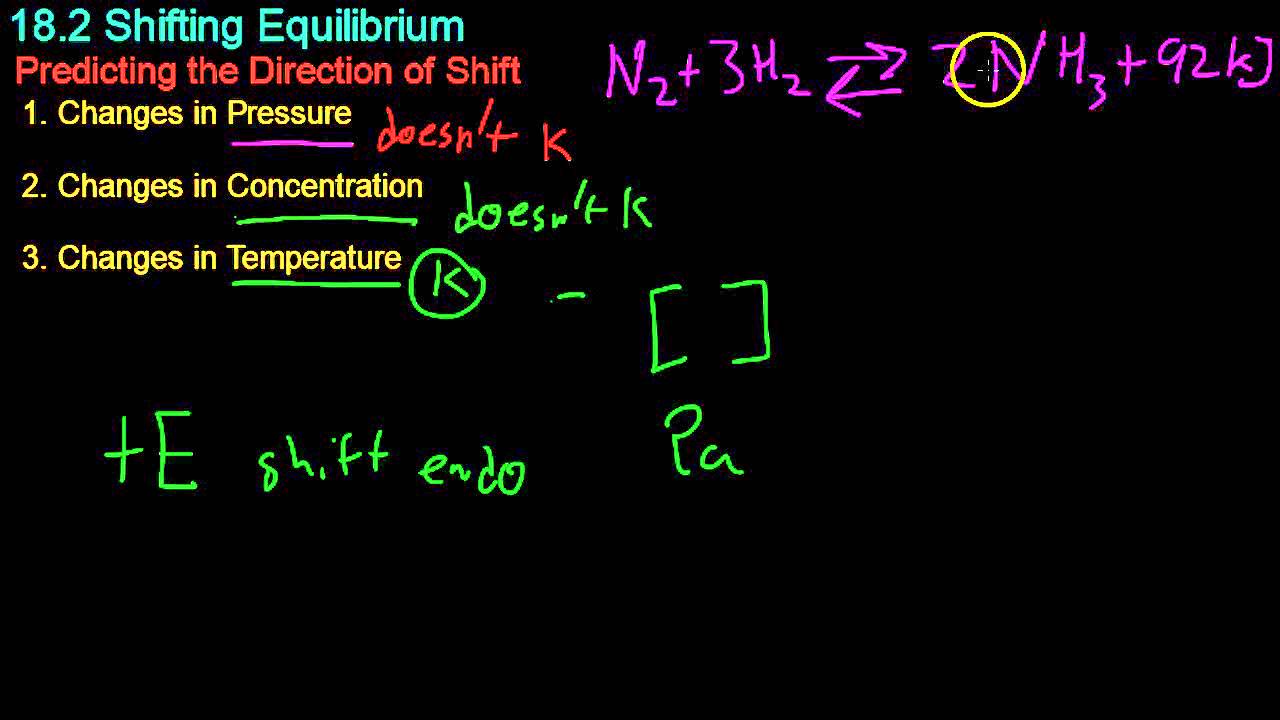
18.2 Shifting Equilibrium

AQA 1.6 Equilibria REVISION

Faktor-Faktor yang Mempengaruhi Pergeseran Kesetimbangan dan Penerapannya dalam Industri
GCSE Chemistry - Le Chatelier's Principle #50 (Higher Tier)

Deslocamento de Equilíbrio - Princípio de Le Chatelier

PERGESERAN KESETIMBANGAN (ASAS LE CHATELIER) : KESETIMBANGAN KIMIA KELAS 11
5.0 / 5 (0 votes)
