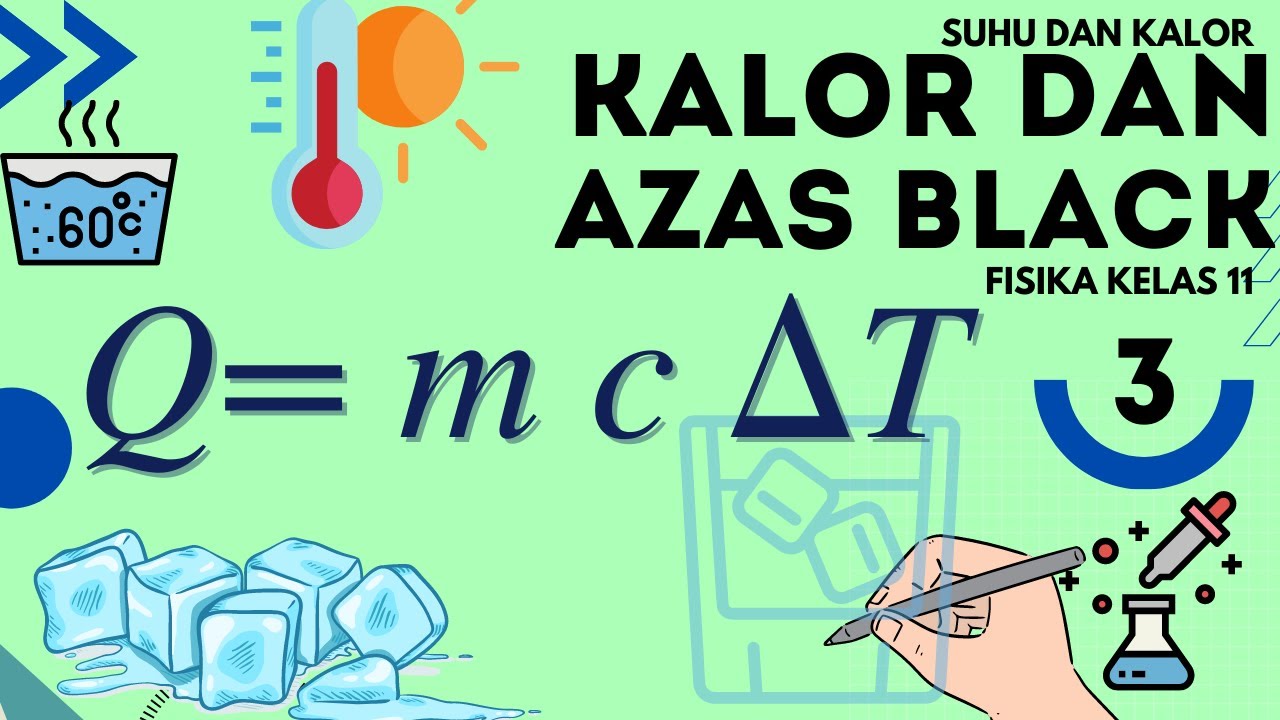CALORIMETRIA: UM SUPER MAPA MENTAL | QUER QUE DESENHE
Summary
TLDRIn this educational video on calorimetry, the host explains the concept of heat transfer, focusing on both sensible and latent heat. Viewers learn how heat flows from a body with a higher temperature to one with a lower temperature, and how to calculate heat involved in different processes. The video covers key concepts like specific heat capacity, latent heat, thermal power, and the principle of heat exchange. The host also emphasizes the importance of understanding these principles for exams and provides helpful resources like a downloadable mental map for studying.
Takeaways
- 😀 Calorimetry is the study of heat energy flow from one body to another.
- 😀 Heat always flows from a body with higher temperature to one with lower temperature.
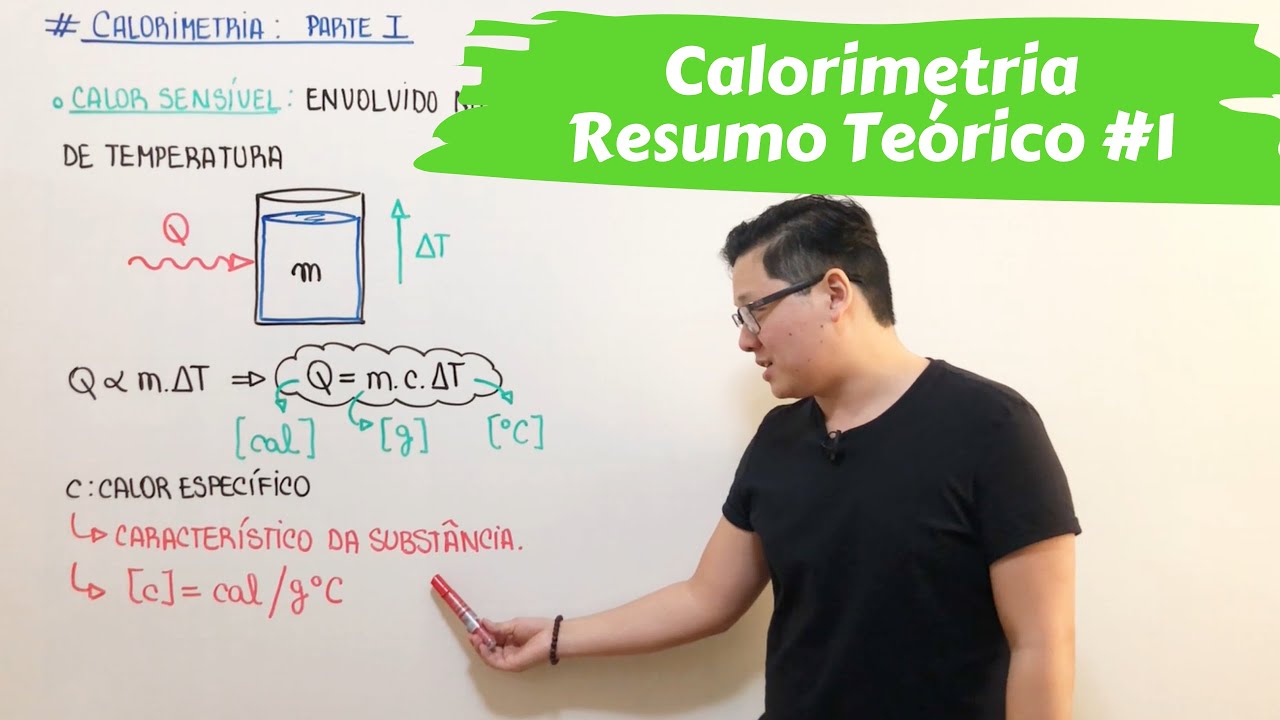
- 😀 Sensible heat refers to the heat flow that causes a temperature change in a body.
- 😀 The formula for calculating sensible heat is: Q = mass × specific heat × temperature change.
- 😀 Latent heat refers to the heat flow that causes a change in the physical state of a substance without changing its temperature.
- 😀 The formula for calculating latent heat is: Q = mass × latent heat of fusion/vaporization.
- 😀 Thermal capacity measures how much heat is required to change the temperature of an entire body.
- 😀 The thermal capacity formula is: Thermal Capacity = mass × specific heat or Thermal Capacity = heat quantity ÷ temperature change.
- 😀 The difference between specific heat (sensible heat) and thermal capacity should be noted carefully.
- 😀 Thermal power represents the rate at which heat is emitted by a thermal source and is calculated as: Power = heat quantity ÷ time.
- 😀 The heat exchange between two bodies can be understood through the principle of heat exchange, where the heat ceded by one body is equal to the heat received by the other body.
Q & A
What is calorimetry?
-Calorimetry is the study of heat, specifically the flow of thermal energy between bodies, transferring from a hotter body to a cooler one.
What is the difference between sensible heat and latent heat?
-Sensible heat refers to the flow of heat that causes a temperature change in a body, while latent heat involves heat that causes a change in the physical state of a substance without changing its temperature.
How is sensible heat calculated?
-Sensible heat can be calculated using the formula: Heat = mass × specific heat × temperature change. The result is measured in calories, with mass in grams, temperature change in Celsius, and specific heat in cal/g°C.
How do you calculate latent heat?
-Latent heat is calculated using the formula: Heat = mass × latent heat of the phase change. The result is measured in calories, with mass in grams.
What is specific heat capacity?
-Specific heat capacity is a physical property that measures how much heat is needed to change the temperature of a substance. It differs from sensible heat, as it relates to a whole body’s temperature change rather than the heat exchange of a substance.
What is the difference between specific heat and heat capacity?
-Specific heat refers to the heat required to raise the temperature of one gram of a substance by one degree Celsius, while heat capacity refers to the total heat required to change the temperature of an entire object or body.
What is the formula for heat capacity?
-The heat capacity can be calculated in two ways: Heat capacity = mass × specific heat, or Heat capacity = heat divided by temperature change.
How is thermal power calculated?
-Thermal power is the amount of heat emitted by a source per unit time and can be calculated using the formula: Power = heat / time. Power is measured in calories per second.
What is thermal equilibrium?
-Thermal equilibrium occurs when two bodies in contact exchange heat until both reach the same temperature. The total heat gained by one body equals the total heat lost by the other.
How does heat transfer between bodies?
-Heat always flows from the body with higher temperature to the body with lower temperature. The rate of heat transfer depends on the thermal properties of the materials involved.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード5.0 / 5 (0 votes)