Física - Calor Sensível e Calor Latente
Summary
TLDRIn this educational video, Professor Jordi explains the fundamental concepts of heat in physics. He clarifies the difference between sensible and latent heat, discussing how heat affects a body either by changing its temperature or its physical state. Using various experiments, the video demonstrates how heat transfer depends on mass, material, and temperature change. Professor Jordi also explains how to calculate heat using formulas like McΔθ for sensible heat and mL for latent heat, and provides a practical exercise involving the heating and melting of ice to further illustrate these concepts.
Takeaways
- 😀 Heat is energy in transit, moving from a hotter object to a cooler one.
- 😀 Heat can have two primary effects on an object: it can change its temperature (sensible heat) or change its physical state (latent heat).
- 😀 The formula for sensible heat is Q = mcΔθ, where Q is the heat, m is the mass, c is the specific heat, and Δθ is the temperature change.
- 😀 Sensible heat involves a change in temperature, which can be calculated based on the heat provided to a body.
- 😀 The specific heat of a material determines how much heat is required to change its temperature.
- 😀 Experimentally, when heat is provided to two objects with identical mass and material, the temperature change is directly proportional to the amount of heat.
- 😀 For two objects with different masses but the same material, a larger mass requires more heat to achieve the same temperature change.
- 😀 Latent heat is the heat required for a substance to change its physical state (like from solid to liquid) without changing its temperature.
- 😀 The formula for latent heat is Q = mL, where Q is the heat, m is the mass, and L is the latent heat of the material.
- 😀 An example exercise demonstrated how to calculate the heat needed to transform 10g of ice at -10°C to water at 40°C, which involves sensible and latent heat calculations, totaling 1250 calories.
Q & A
What is the definition of heat according to the script?
-Heat is defined as energy in transit, which always moves from a body with a higher temperature to a body with a lower temperature.
Why do we use the symbol 'θ' (theta) for temperature instead of 'T'?
-The symbol 'θ' is used for temperature to avoid confusion with 'T', which is commonly used for time in physics.
What does the formula 'q = McΔθ' represent in the context of heat?
-The formula 'q = McΔθ' calculates the amount of heat involved in a temperature change, where 'q' is the heat, 'M' is the mass of the object, 'c' is the specific heat, and 'Δθ' is the change in temperature.
What is the significance of specific heat in heat calculations?
-Specific heat is a property of a material that indicates the amount of heat required to change the temperature of a unit mass by one degree Celsius. It varies between different materials.
How do the mass and specific heat of an object affect the amount of heat required?
-The greater the mass of an object, the more heat is needed to achieve the same temperature change. Similarly, the specific heat varies by material, affecting how much heat is required for temperature changes.
What happens when a body undergoes a phase change, such as melting or vaporization?
-When a body undergoes a phase change, its temperature does not change. The heat provided is used to alter the physical state, not to increase the temperature.
What is latent heat, and how is it calculated?
-Latent heat is the heat required to change the state of a substance without changing its temperature. It is calculated using the formula 'q = mL', where 'm' is the mass undergoing the phase change and 'L' is the latent heat for that particular phase change.
How does latent heat differ from sensible heat?
-Latent heat involves changes in physical state without temperature change, while sensible heat involves temperature changes without altering the physical state.
What are the key factors that affect the amount of heat required during phase changes?
-The key factors include the mass of the substance undergoing the phase change and the specific latent heat associated with the particular phase change, such as fusion or vaporization.
What is the total heat required to transform 10g of ice at -10°C into water at 40°C?
-The total heat required is 1250 calories, calculated by summing three components: sensible heat to warm ice from -10°C to 0°C, latent heat to melt the ice, and sensible heat to heat the water from 0°C to 40°C.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Calorimetria - Aula 01 (Calor sensível)

EQUAÇÃO FUNDAMENTAL DA ONDULATÓRIA ONDULATÓRIA AULA 2
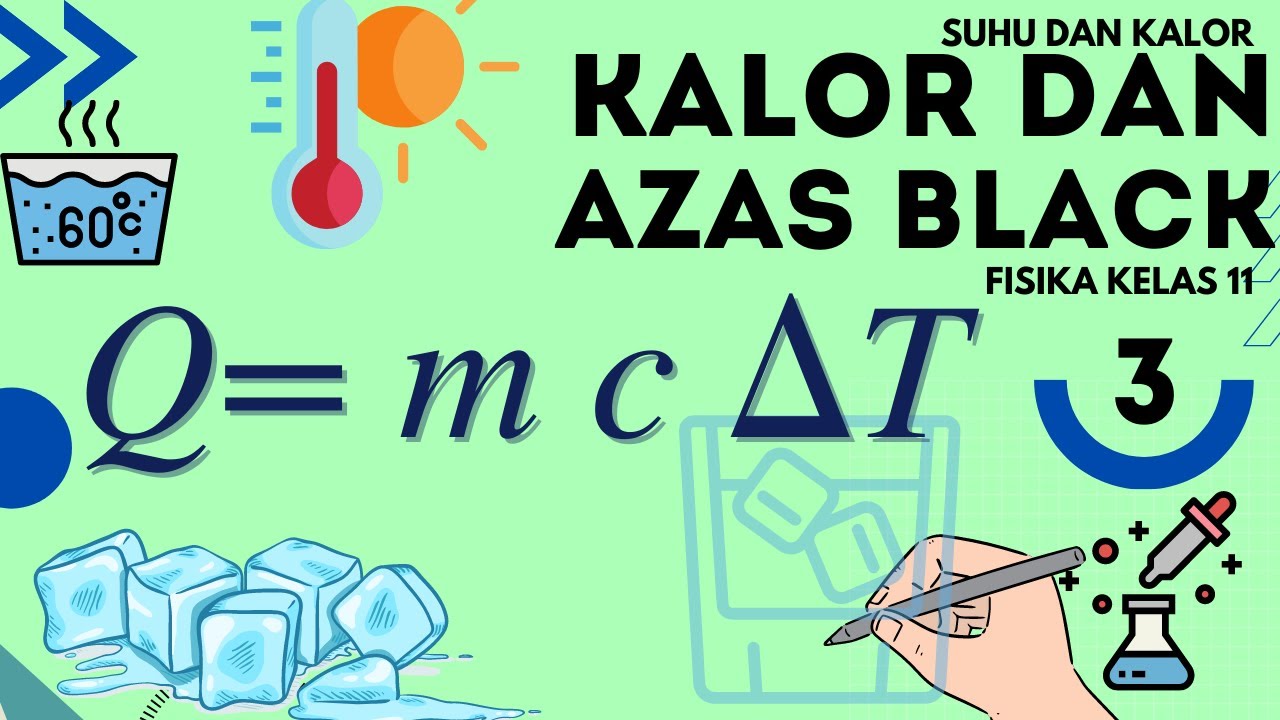
Suhu dan Kalor Fisika Kelas 11 - Part 3 : Kalor dan Azas Black

TROCAS DE CALOR SEM MUDANÇA DE FASE - TERMOLOGIA - Aula 7 - Prof. Boaro

Calor Específico |Termodinámica| Ejercicio - Salvador FI

TROCAS DE CALOR COM MUDANÇA DE FASE - TERMOLOGIA - Aula 8 - Prof. Boaro
5.0 / 5 (0 votes)