1.2 Bonding and 1.3 Structures - Covalent Bonding & Structures
Summary
TLDRThis video explains covalent bonding, where non-metal atoms share electrons to achieve full outer electron shells, forming stable molecules. It covers the formation of single, double, and triple bonds, with examples like fluorine, oxygen, and water. The script also distinguishes between simple covalent and giant covalent structures, with a focus on carbon's allotropes (diamond, graphite, and graphene). Simple covalent molecules have low melting points and do not conduct electricity, while giant covalent structures are hard, have high melting points, and exhibit unique electrical properties, especially graphite and graphene.
Takeaways
- 😀 Covalent bonding occurs when non-metal atoms share electrons to achieve a full outer shell.
- 😀 A covalent bond is defined as a shared pair of electrons between two atoms.
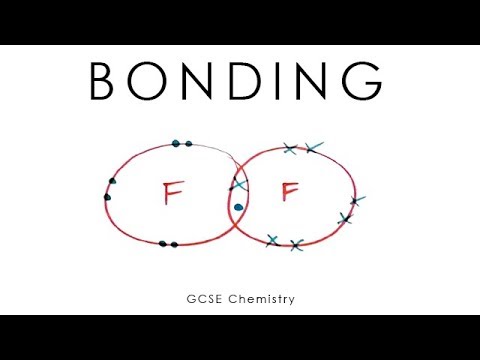
- 😀 Fluorine atoms, which need one electron to complete their outer shell, can form a covalent bond by sharing electrons with another fluorine atom.
- 😀 Oxygen atoms can form a double covalent bond by sharing two pairs of electrons to achieve a stable outer shell.
- 😀 Nitrogen can form triple bonds by sharing three pairs of electrons, creating even stronger bonds.
- 😀 A molecule is two or more atoms covalently bonded together, with diatomic molecules consisting of just two atoms.
- 😀 Lone pairs of electrons are those that are not involved in bonding, unlike shared pairs that form covalent bonds.
- 😀 Simple covalent structures consist of individual molecules that are weakly bonded by intermolecular forces, such as van der Waals forces.
- 😀 Simple covalent molecules generally have low melting and boiling points because only weak intermolecular forces need to be broken.
- 😀 Giant covalent structures, like diamond, graphite, and graphene, involve strong covalent bonds and are associated with higher melting points, hardness, and conductivity (in some cases).
- 😀 Carbon forms giant covalent structures with multiple bonds, creating unique materials like diamond (hard), graphite (soft but conductive), and graphene (flexible and conductive).
Q & A
What is covalent bonding?
-Covalent bonding occurs when non-metal atoms share electrons to achieve a full outer shell, stabilizing both atoms involved in the bond.
What is a key feature of a covalent bond?
-A covalent bond is a shared pair of electrons between two atoms, which results in a strong bond that takes a lot of energy to break.
How does a covalent bond differ from ionic bonding?
-In ionic bonding, electrons are transferred between a metal and a non-metal, whereas in covalent bonding, electrons are shared between non-metal atoms.
What is the definition of a molecule in terms of covalent bonding?
-A molecule is two or more atoms covalently bonded together. For example, fluorine and oxygen molecules are examples of covalent molecules.
What is a diatomic molecule?
-A diatomic molecule is a molecule consisting of exactly two atoms bonded together. Examples include fluorine (F2) and oxygen (O2).
What are lone pairs of electrons?
-Lone pairs are pairs of electrons that are not involved in bonding and are found in the outer shell of an atom.
What are the two types of covalent structures?
-The two types of covalent structures are simple covalent and giant covalent structures.
What are the properties of simple covalent structures?
-Simple covalent structures typically have low melting and boiling points, do not conduct electricity, and are generally not soluble in water.
Why does carbon form giant covalent structures?
-Carbon forms giant covalent structures because it can form four covalent bonds with other atoms, creating large, stable networks such as diamond, graphite, and graphene.
How does the structure of graphite differ from that of diamond?
-In graphite, each carbon is bonded to three others, forming layers of hexagonal rings with weak forces between layers, allowing them to slide. In diamond, each carbon is bonded to four others, forming a rigid, three-dimensional structure, making it hard and having a high melting point.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

Chemical Bonding Explained | Ionic, Covalent and Metallic | GCSE Chemistry

Types Of Chemical Bonds - What Are Chemical Bonds - Covalent Bonds And Ionic Bonds - What Are Ions

Regra do Octeto

Atoms to Ions | Atom | Lesson 6 | National 5 Chemistry

Chemical Bonding: Covalent Bonding Lewis Dot Diagrams
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry - long version
5.0 / 5 (0 votes)
