REACCIONES ORGANICAS DE COMBUSTIÓN | Ejercicios con Alcanos
Summary
TLDRIn this video, Emmanuel from 'Asesorías Emmanuel' guides viewers through balancing chemical combustion reactions. He explains that combustion reactions typically involve hydrocarbons and oxygen, producing carbon dioxide and water. The video focuses on balancing chemical equations by adjusting the number of carbon, hydrogen, and oxygen atoms. Emmanuel demonstrates multiple examples, highlighting step-by-step adjustments and how to use coefficients, including decimals when necessary, to balance complex equations. The video is designed to help viewers grasp the core concepts of combustion reactions in organic chemistry. Greetings are also extended from Mexico, encouraging viewers to subscribe.
Takeaways
- 🇲🇽 Greeting from Mexico and invitation to subscribe to Emmanuel's educational channel.
- 🔬 Introduction to a chemistry lesson focused on combustion reactions.
- ➡️ Explanation of chemical equations: reactants are on the left, products are on the right.
- 🔥 Combustion reactions involve hydrocarbons (compounds of carbon and hydrogen) and oxygen.
- 📜 Common combustion products: carbon dioxide (CO₂) and water (H₂O).
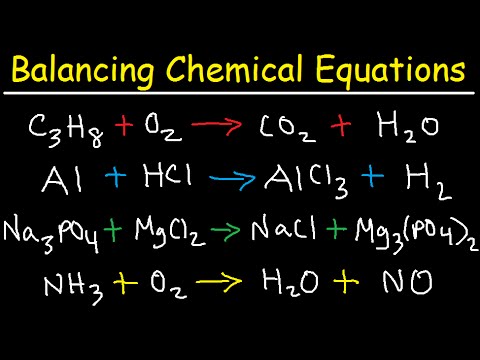
- 🔄 Balancing chemical equations involves ensuring equal numbers of each atom on both sides.
- ✍️ Step-by-step example of balancing a reaction: Start by balancing carbon, then hydrogen, and finally oxygen.
- 🧪 Emphasis on the use of whole numbers and decimals in balancing, with an example using 6.5 for oxygen.
- 🧩 Combustion of organic compounds will consistently produce CO₂ and H₂O as products.
- 📏 Practice examples: Tips on organizing atoms in lists to systematically balance complex reactions.
Q & A
What is a chemical reaction, and how is it identified?
-A chemical reaction is a process where substances (reactants) are transformed into different substances (products). It is identified by the presence of an arrow in the equation, where the reactants are on the left and the products are on the right.
What is a combustion reaction?
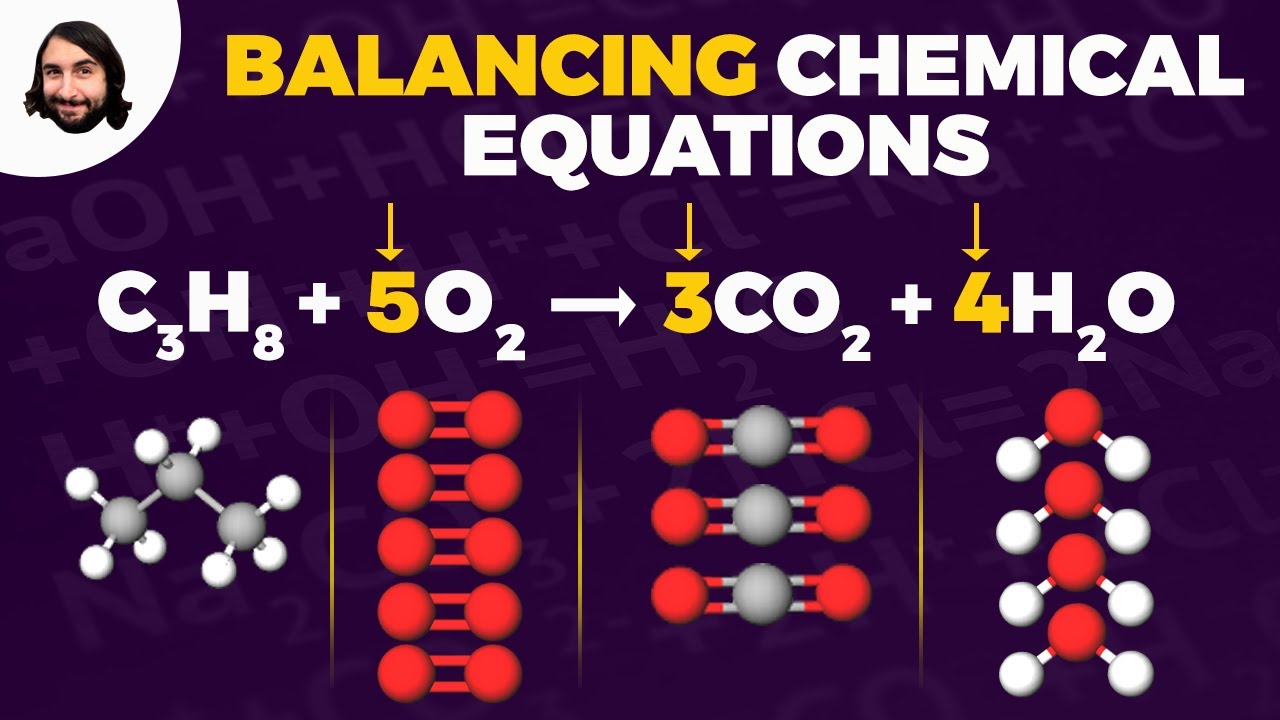
-A combustion reaction occurs when a hydrocarbon reacts with oxygen, producing carbon dioxide (CO2) and water (H2O). It always involves carbon and hydrogen (from the hydrocarbon) reacting with oxygen.
How do you identify a hydrocarbon in a combustion reaction?
-A hydrocarbon is identified by the presence of carbon (C) and hydrogen (H) atoms in the compound. In combustion reactions, these are the key components that react with oxygen.
Why is balancing a chemical equation important?
-Balancing a chemical equation is crucial because it ensures that the same number of atoms for each element are present on both sides of the equation, obeying the law of conservation of mass.
How is the number of carbon atoms balanced in a combustion reaction?
-To balance carbon atoms, you compare the number of carbon atoms on the left (reactants) with those on the right (products). If there are more carbon atoms on one side, you adjust the coefficient of the compound containing carbon on the other side.
What strategy is used to balance hydrogen atoms in a combustion reaction?
-To balance hydrogen atoms, count the number of hydrogens in the reactants (typically in the hydrocarbon) and adjust the coefficient of water (H2O) on the product side to ensure the same number of hydrogen atoms.
How are oxygen atoms balanced in a combustion reaction?
-Oxygen atoms are balanced by first counting the total oxygen atoms in both CO2 and H2O on the product side. Then, adjust the coefficient of O2 on the reactant side to match the total number of oxygen atoms.
What is the significance of using decimals, such as 6.5, in balancing equations?
-Decimals like 6.5 can be used when balancing oxygen atoms to achieve the exact number needed. Multiplying the decimal by 2 will give a whole number for oxygen, ensuring the equation is balanced.
What is the typical formula for a combustion reaction?
-The typical formula for a combustion reaction is: hydrocarbon + O2 → CO2 + H2O. This represents a hydrocarbon reacting with oxygen to produce carbon dioxide and water.
Why are coefficients used in balancing chemical equations?
-Coefficients are used to adjust the number of molecules or atoms in the equation without changing the chemical identity of the substances involved, ensuring the equation is balanced in terms of atoms.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード5.0 / 5 (0 votes)