Photoelectric Effect Theory Lesson
Summary
TLDRThis educational script delves into the photoelectric effect, emphasizing the particle nature of light. It outlines the electromagnetic spectrum, detailing the relationship between frequency, wavelength, and energy of photons. The script explains how photons with higher frequency possess more energy, behaving more like particles, particularly with gamma rays. It introduces Planck's constant and the equations governing the photoelectric effect, including the work function and kinetic energy of emitted electrons. The threshold frequency for electron ejection and the impact of light intensity on photocells are also discussed, providing a comprehensive overview of the fundamental principles of quantum physics.
Takeaways
- 🌌 The electromagnetic spectrum consists of seven parts, ranging from gamma rays to radio waves, with gamma rays having the highest frequency and radio waves the lowest.
- 🔄 All electromagnetic waves are transverse waves that travel at the speed of light (3 x 10^8 m/s) in a vacuum and carry energy in the form of photons.
- ⚡ The energy of photons is directly proportional to the frequency of the electromagnetic waves, with gamma rays having the highest energy due to their high frequency.
- 📉 Photons with higher frequencies exhibit more particle-like behavior, while those with lower frequencies show more wave-like properties.
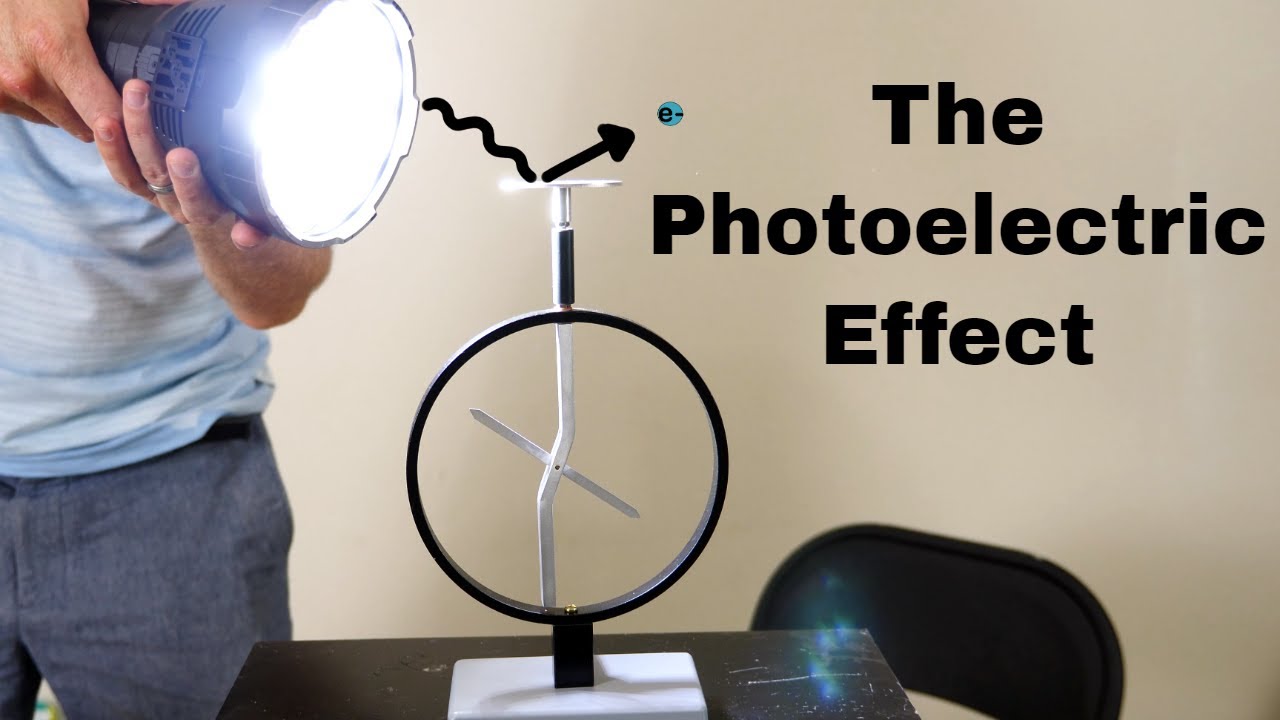
- 🔬 The photoelectric effect demonstrates the particle nature of light, where electrons are ejected from a metal surface when struck by light of a suitable frequency.
- ⚡️ Each metal has a specific threshold frequency, which is the minimum frequency required for the photoelectric effect to occur, and is directly related to the work function of the metal.
- 📉 The kinetic energy of emitted electrons (photoelectrons) is directly related to the frequency of the incident light, following the equation EK = hf - W0, where EK is the kinetic energy, hf is the energy of the photon, and W0 is the work function.
- 📊 The graph of kinetic energy versus frequency for emitted electrons is a straight line with a slope equal to Planck's constant, and the y-intercept representing the work function of the metal.
- 🔑 The work function and threshold frequency of a metal are indicators of how tightly electrons are bound to the metal's atoms, with higher values meaning electrons are more tightly bound.
- 🔆 The intensity of incident light affects the number of photoelectrons emitted, leading to a stronger current in a photocell, while the frequency of the light affects the kinetic energy of the emitted electrons.
Q & A
What are the seven parts of the electromagnetic spectrum?
-The electromagnetic spectrum consists of gamma rays, x-rays, ultraviolet, visible light, infrared, microwaves, and radio waves.
What is the relationship between wavelength and frequency in electromagnetic waves?
-The frequency of electromagnetic waves is inversely proportional to their wavelength. The shorter the wavelength, the higher the frequency, and vice versa.
How does the energy of photons relate to the frequency of electromagnetic waves?
-The energy of photons is directly proportional to the frequency of the electromagnetic waves. Higher frequency waves carry photons with greater energy.
What is the significance of Planck's constant in the context of photon energy?
-Planck's constant (6.63 x 10^-34 joules seconds) is used in the equation E = h * f to calculate the energy of a photon, where E is the energy, h is Planck's constant, and f is the frequency of the electromagnetic wave.
What is the photoelectric effect and its significance in understanding the particle nature of light?
-The photoelectric effect is the emission of electrons from a metal surface when light of a suitable frequency shines on it. It demonstrates the particle nature of light, as electrons are ejected due to the energy of individual photons.
What is the threshold frequency, and how does it relate to the photoelectric effect?
-The threshold frequency is the minimum frequency of light required to eject electrons from a metal surface. If the frequency of the incident light is higher than this threshold, electrons will be emitted.
How is the work function of a metal related to its threshold frequency?
-The work function of a metal is the minimum energy needed for an electron to be emitted from the metal surface. It is calculated using the equation W0 = h * f0, where W0 is the work function, h is Planck's constant, and f0 is the threshold frequency.
What is the kinetic energy of photoelectrons in relation to the frequency of incident light?
-The kinetic energy of photoelectrons (EK) is given by the equation EK = hf - W0, where hf is the energy of the incident photon and W0 is the work function of the metal. The higher the frequency of the incident light, the greater the kinetic energy of the emitted electrons, provided the frequency is above the threshold frequency.
How does the intensity of incident light affect the photoelectric effect?
-An increase in the intensity of incident light increases the number of photons striking the metal, which in turn increases the number of electrons emitted and the current strength in a photocell, but it does not affect the kinetic energy of the individual photoelectrons.
What can be inferred about the binding energy of electrons in metals from their threshold frequencies?
-Metals with higher threshold frequencies require more energy to eject electrons, indicating that electrons are more tightly bound to the metal's atoms. Conversely, metals with lower threshold frequencies have electrons that are more loosely bound and can be ejected with less energy.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

The Double-Slit Experiment
Knocking Electrons With Light—The Photoelectric Effect
Electromagnetic Radiation and Electromagnetic Spectrum | X-ray physics | Radiology Physics Course #7

#Fisikapopuler Eps. 1: Sejarah Lahirnya Fisika Kuantum

Dual Nature of Radiation & Matter in 10 mins 😱🔥 Ch 11 Physics Class 12 Boards 2022-23 Score 95+

Albert Einstein and The Photoelectric Effect | AMS OpenMind
5.0 / 5 (0 votes)
