Heating Curve Grade 10: Kinetic Molecular Theory
Summary
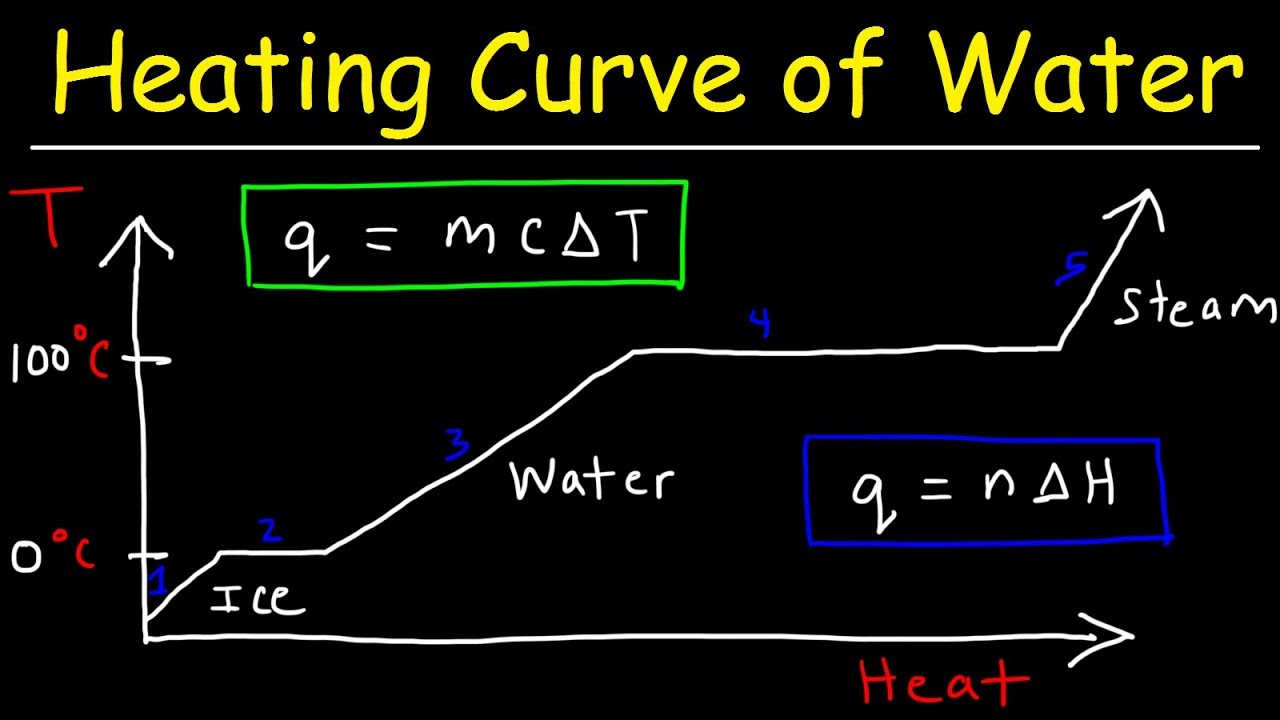
TLDRIn this educational video, Miss Martins explores the kinetic molecular theory's application to heating curves, emphasizing the distinction between heat energy and temperature. She explains that adding heat doesn't always raise a substance's temperature, highlighting phase changes where heat energy is used to overcome intermolecular forces without increasing temperature. The video uses the heating curve of water as an example, illustrating how temperature remains constant during melting and boiling, and encourages viewers to understand the kinetic theory's implications for phase changes.
Takeaways
- 🔥 The kinetic molecular theory explains the behavior of particles in a substance when heat is applied.
- ⏱️ A heating curve is a graphical representation of the temperature change of a substance over time during heating.
- ⚠️ Adding heat to a substance does not always result in a temperature increase; the energy can be used for phase changes.
- 📊 The flat parts of a heating curve indicate a phase change is occurring, where energy is used to overcome intermolecular forces without increasing temperature.
- 💧 The heating curve of water shows distinct phases: solid (ice), liquid, and gas, with phase changes at specific temperatures.
- 🌡️ The melting point and boiling point of a substance are represented by the horizontal sections of the heating curve, where temperature remains constant during phase change.
- 📉 On the heating curve, the inclined parts represent periods where the substance is in a single phase and its temperature is increasing due to added heat.
- 🔍 The axes of a heating curve are crucial, with the y-axis representing temperature in degrees Celsius and the x-axis representing time in minutes.
- 🌟 The kinetic molecular theory states that when a substance is heated, it can either increase in temperature (if in the same phase) or undergo a phase change without a temperature increase.
- 🔄 During phase changes, such as melting (solid to liquid) or boiling/evaporation (liquid to gas), both phases coexist, and the added heat energy is used to weaken intermolecular forces.
Q & A
What is the kinetic molecular theory?
-The kinetic molecular theory is a concept that explains the behavior of particles in a substance when energy is added or removed. It relates to how substances change phases and how their temperature changes with the addition of heat energy.
What is a heating curve and how is it represented?
-A heating curve is a graphical representation that shows the change in temperature of a substance over time as heat is added. It typically has time on the x-axis and temperature on the y-axis.
Why doesn't the temperature of a substance always increase when heat is added?
-The temperature of a substance doesn't always increase when heat is added because the energy can be used for phase changes, such as melting or boiling, where the intermolecular forces are overcome without increasing the temperature.
What is the significance of the flat parts of a heating curve?
-The flat parts of a heating curve indicate that a phase change is occurring. During these periods, the substance absorbs heat energy to change its state without a rise in temperature.
What are the two possible outcomes when heat is added to a substance according to the kinetic molecular theory?
-When heat is added to a substance, the kinetic molecular theory suggests two possible outcomes: an increase in temperature due to increased average kinetic energy, or no increase in temperature as the energy is used to overcome intermolecular forces during a phase change.
Why is it important to label the axes of a heating curve correctly?
-Labeling the axes of a heating curve correctly is important for clarity and accuracy. It ensures that the graph is easily understood, with the y-axis typically representing temperature and the x-axis representing time.
What is the difference between the inclined and horizontal parts of a heating curve?
-The inclined parts of a heating curve represent periods where the substance is in a single phase and its temperature is increasing due to added heat. The horizontal parts indicate phase changes, where the substance is transitioning from one state to another without a change in temperature.
What is the melting point and boiling point of water, and how do they relate to the heating curve?
-The melting point of water is around 0 degrees Celsius, and the boiling point is around 100 degrees Celsius. These points are significant on the heating curve as they mark the transition from solid to liquid (melting point) and liquid to gas (boiling point).
How can you identify the phase or phases present during different parts of a heating curve?
-You can identify the phase or phases present during different parts of a heating curve by observing the inclined and horizontal sections. Inclined sections indicate a single phase, while horizontal sections indicate a phase change where two phases coexist.
What is the role of intermolecular forces in the context of a heating curve?
-Intermolecular forces play a crucial role in a heating curve as they resist changes in the state of a substance. During phase changes, the added heat energy is used to weaken these forces, allowing the substance to transition from one phase to another without an immediate increase in temperature.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

Kinetic Molecular Theory grade 10 Introduction

2.5 Heating/Cooling Curves (Potential and Kinetic Energy Changes)

Cooling curve vs Heating curve Grade 10 Chemistry
Heating Curve and Cooling Curve of Water - Enthalpy of Fusion & Vaporization

lesson 7 4 calculating heat of phase changes

Difference Between Temperature and Heat
5.0 / 5 (0 votes)
