Termodinamika: Hukum 0 Termodinamika
Summary
TLDRIn this video, Tommy explains the concept of the Zeroth Law of Thermodynamics, using the example of making coffee. He describes how heat transfers from the coffee to the cup, and from the cup to the surrounding air, leading to thermal equilibrium between the coffee, cup, and air. He then uses a simple example of a mercury thermometer to further illustrate the concept of thermal equilibrium. Tommy emphasizes that when two systems are in thermal equilibrium with a third system, they are also in equilibrium with each other. The video ends with an invitation for viewers to ask questions in the comments.
Takeaways
- 😀 The speaker, Tommy, introduces the topic of the Zeroth Law of Thermodynamics in a casual and engaging manner.
- 😀 The Zeroth Law of Thermodynamics explains thermal equilibrium, stating that if two systems are in thermal equilibrium with a third system, they are in thermal equilibrium with each other.
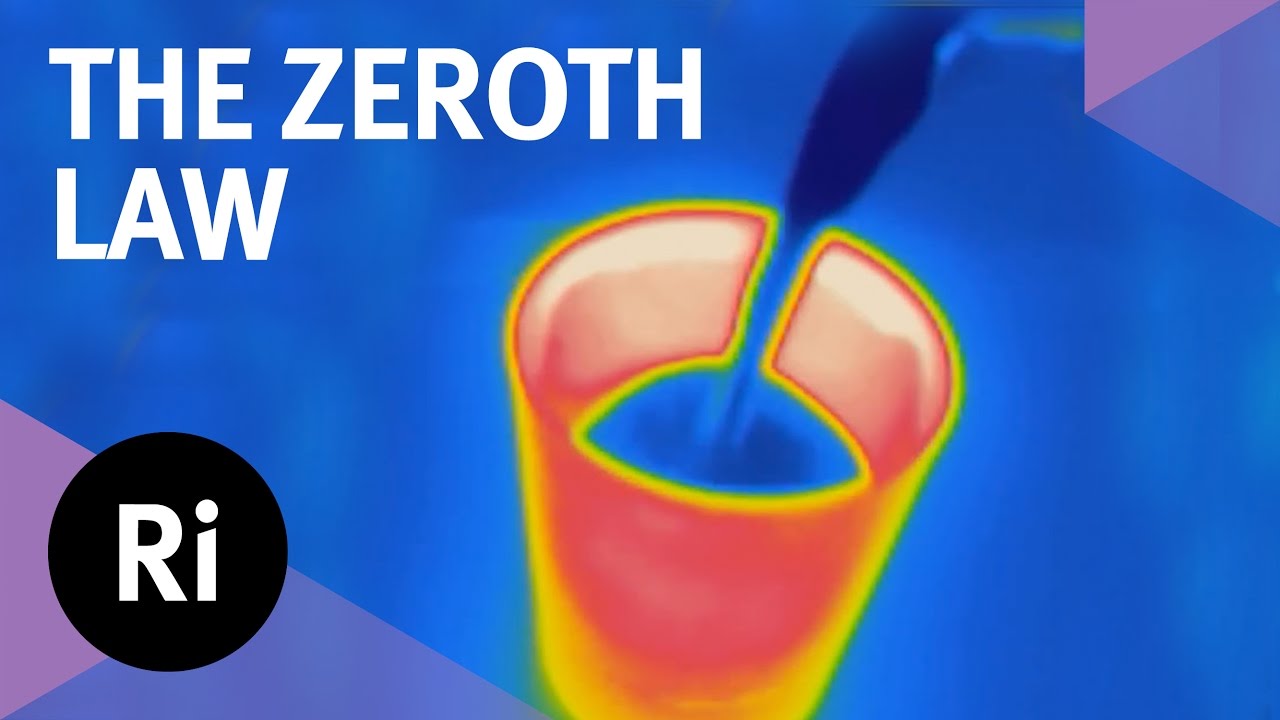
- 😀 An example of thermal equilibrium is given using a cup of hot coffee. The heat from the coffee will transfer to the cup and the surrounding air, leading to a drop in the coffee's temperature over time.
- 😀 The temperature of the coffee will eventually equal the temperature of the surrounding air and the cup, illustrating the concept of thermal equilibrium.
- 😀 The script emphasizes that this process is an example of the Zeroth Law of Thermodynamics in action, where energy transfers between systems until thermal equilibrium is achieved.
- 😀 Tommy invites viewers to visualize the concept by imagining the coffee cooling down and transferring heat to the surrounding environment.
- 😀 A practical example involving a mercury thermometer is used to further explain thermal equilibrium. When the thermometer is placed on skin, heat from the skin transfers to the thermometer, causing the mercury to expand.
- 😀 This mercury thermometer example demonstrates how the body (skin) and the thermometer reach thermal equilibrium, with the temperature of the thermometer matching that of the skin.
- 😀 The Zeroth Law of Thermodynamics is important because it helps define temperature and the way different objects interact thermally.
- 😀 Tommy encourages viewers to comment if they have questions or confusion, fostering engagement and interaction in the learning process.
Q & A
What is the main concept being discussed in the video?
-The main concept being discussed is the Zeroth Law of Thermodynamics, which explains thermal equilibrium between systems.
How is the Zeroth Law of Thermodynamics explained through the example of making coffee?
-The example illustrates that when hot coffee is left to cool, its temperature gradually decreases and eventually matches the temperature of the cup and the surrounding air, indicating thermal equilibrium between the coffee, cup, and air.
What happens to the temperature of the coffee after it is made?
-The temperature of the coffee decreases over time, transferring heat to the cup and the surrounding air, until it reaches thermal equilibrium with the cup and air.
What role does the surrounding air play in the coffee cooling process?
-The surrounding air absorbs some of the heat from the coffee, causing the air temperature to increase while the coffee cools down.
What does it mean when the temperatures of the coffee, cup, and air match?
-It means the systems have reached thermal equilibrium, where no heat is flowing between them, which is an example of the Zeroth Law of Thermodynamics in action.
How does the example of using a thermometer on skin relate to the Zeroth Law?
-The thermometer in contact with the skin absorbs heat from the skin, and the mercury inside the thermometer expands until it reaches the same temperature as the skin, demonstrating thermal equilibrium between the skin and thermometer.
Why does the mercury inside the thermometer expand when in contact with skin?
-The mercury expands as it absorbs heat from the skin, which causes the temperature of the mercury to rise until it matches the skin temperature.
What is meant by 'thermal equilibrium' in the context of the Zeroth Law?
-Thermal equilibrium occurs when two or more systems are at the same temperature, meaning no heat flows between them. This concept is central to the Zeroth Law of Thermodynamics.
How does the Zeroth Law of Thermodynamics relate to real-world objects?
-In real-world applications, the Zeroth Law helps us understand how objects like coffee, thermometers, and even the air around us interact in terms of temperature and heat transfer.
Why is it important to understand the Zeroth Law of Thermodynamics?
-Understanding the Zeroth Law is fundamental in fields like physics and engineering, as it forms the basis for concepts like temperature measurement and heat flow between systems.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantVoir Plus de Vidéos Connexes

THE LAWS OF THERMODYNAMICS (Zeroth law, 1st, 2nd and 3rds law) | ENGINEERING THERMODYNAMICS |

ZEROTH LAW OF THERMODYNAMICS | Simple & Basic Animation

Termodinamika: Cabang Fisika yang Luar Biasa

The Zeroth Law of Thermodynamics: Thermal Equilibrium

The First & Zeroth Laws of Thermodynamics: Crash Course Engineering #9
What is the Zeroth Law of Thermodynamics?
5.0 / 5 (0 votes)
