What is Infrared Radiation & Electromagnetic Spectrum? - [4]
Summary
TLDRIn this episode of '10 Minute Science,' viewers are introduced to the concept of infrared radiation, a type of electromagnetic wave associated with heat. The video explains how infrared radiation was discovered by William Herschel, who found that the temperature increased beyond the red spectrum, indicating the presence of invisible light. It also covers the electromagnetic spectrum, the relationship between wavelength, frequency, and energy, and how infrared radiation is used to detect heat, even in the dark. The script concludes by pondering the unknown aspects of the universe that remain undiscovered due to our limited sensory and technological capabilities.
Takeaways
- 🌡️ Infrared radiation is associated with heat and is radiated by all objects, even those not visibly glowing.
- 👀 Infrared light is invisible to the human eye but can be detected with specialized cameras or instruments.
- 🏠 The script uses images to demonstrate how infrared radiation can reveal heat leaks in a house and the relative temperature of different areas.
- 🤒 Infrared radiation can be used to detect fever in humans, as an elevated body temperature emits more infrared radiation.
- 🔬 The electromagnetic spectrum includes a range of wavelengths, with infrared lying just beyond the red visible light.
- 🌈 The visible light spectrum is just a small part of the entire electromagnetic spectrum, which also includes radio waves, microwaves, and gamma rays.
- 🔍 The discovery of infrared radiation by William Herschel was accidental, as he noticed higher temperatures beyond the red light when using a prism and thermometer.
- ⚫ The black body curve illustrates how objects radiate energy at different wavelengths according to their temperature, with infrared often being more prevalent than visible light.
- 🔆 Even though violet light has higher energy photons, the sun emits fewer violet photons than red or infrared, affecting the temperature readings in Herschel's experiment.
- 🔬 Quantum mechanics tells us that the energy of a photon is related to its frequency, with higher frequencies corresponding to higher energies.
- 🌌 The script ends with a thought-provoking question about the unknown aspects of the universe that we may be unaware of due to our limited sensory and technological detection capabilities.
Q & A
What is infrared radiation?
-Infrared radiation is a type of electromagnetic radiation that we associate with heat. It is invisible to the human eye but can be detected with special cameras and is emitted by all objects with a temperature above absolute zero.
How can infrared radiation be visualized?
-Infrared radiation can be visualized using special cameras that are sensitive to infrared wavelengths. These cameras can create images that represent the relative heat of objects, with colors such as red indicating hotter areas and blue indicating cooler areas.
What does the color in an infrared image represent?
-In an infrared image, different colors represent the relative temperature of the objects being imaged. Reddish colors indicate hotter areas, while bluer and yellow colors represent cooler areas.
How was infrared radiation discovered?
-Infrared radiation was discovered by William Herschel in 1800. He found that there was a type of invisible light beyond the red end of the visible spectrum that caused a thermometer to register higher temperatures than the visible light.
What is the electromagnetic spectrum?
-The electromagnetic spectrum is a range of all types of electromagnetic radiation, arranged by wavelength or frequency. It includes radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays.
Why can't we see infrared radiation with our eyes?
-We can't see infrared radiation with our eyes because the chemistry of our eyes is sensitive only to a specific range of wavelengths known as the visible spectrum. Infrared wavelengths are longer than the red light that marks the limit of our visible range.
What is the relationship between wavelength, frequency, and energy of electromagnetic waves?
-The relationship between wavelength, frequency, and energy is such that as the wavelength gets longer, the frequency gets lower and the energy of the photons decreases. Conversely, as the wavelength gets shorter, the frequency gets higher and the energy of the photons increases.
How does the sun emit different frequencies of light?
-The sun emits light across a wide range of frequencies. However, it does not emit all frequencies equally. The distribution of frequencies emitted by the sun follows a pattern known as a blackbody curve, which has a peak and then gradually decreases on either side.
Why does a thermometer measure higher temperatures in the infrared region compared to violet light, even though violet photons have higher energy?
-Although violet photons have higher energy than infrared photons, the sun emits fewer violet photons than it does red or infrared photons. The temperature measured by a thermometer is influenced by the total energy from all photons hitting it, not just the energy of individual photons.
What is the significance of the discovery of infrared radiation in relation to our understanding of the universe?
-The discovery of infrared radiation expanded our understanding of the universe by revealing that there is more to the electromagnetic spectrum than what is visible to the human eye. It also highlighted the fact that objects emit radiation based on their temperature, which is crucial for technologies such as thermal imaging and understanding celestial bodies.
What are some applications of infrared technology that we use today?
-Infrared technology is used in various applications today, including thermal imaging for detecting heat signatures, night vision for military and security purposes, remote controls for electronics, and in astronomy for studying stars and other celestial objects.
How can the concept of infrared radiation be used to detect fever in humans?
-Infrared radiation can be used to detect fever in humans by measuring the heat emitted by the body. When a person has a fever, their body temperature increases, and so does the amount of infrared radiation they emit. Specialized infrared cameras can capture this increased radiation, indicating a fever.
What is the connection between infrared radiation and the study of dark matter and dark energy?
-The connection between infrared radiation and the study of dark matter and dark energy is that they all represent aspects of the universe that are not directly observable with our senses or current instruments. The discovery of infrared radiation, which was initially undetected, has led to the exploration of other phenomena that may be present but currently beyond our detection capabilities.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantVoir Plus de Vidéos Connexes

Electromagnetic waves: definition, types, benefits and harms ||Physics
Electromagnetic Radiation and Electromagnetic Spectrum | X-ray physics | Radiology Physics Course #7

Lesson 3: Uses and Effects of EM Waves

USES OF ELECTROMAGNETIC WAVES
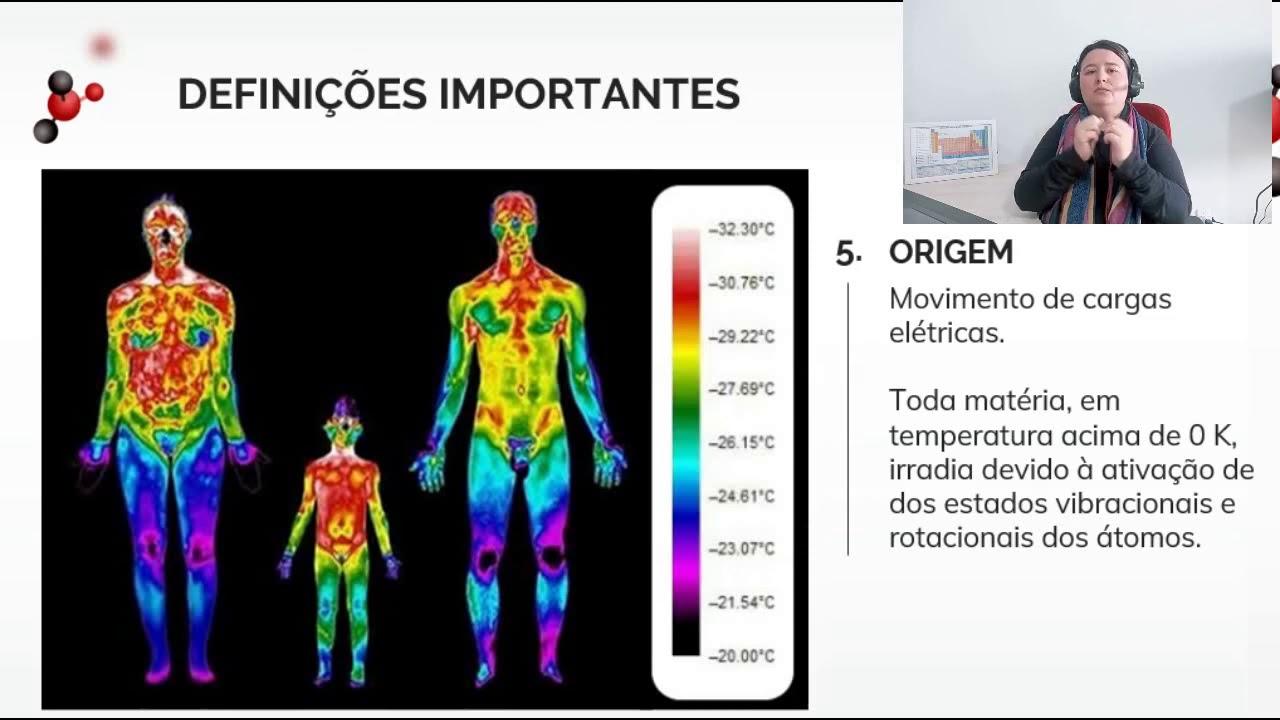
3.2.2 - O que é radiação eletromagnética?

Electromagnetic Spectrum-Grade 10 Waves, Sound and Light- Lesson 7
5.0 / 5 (0 votes)
