Making YOU the Scientist: Freezing Point Depression and Phase Changes
Summary
TLDRThis educational video demonstrates how to freeze water without a freezer by leveraging the principles of freezing point depression and phase changes. The experiment involves creating an ice-water slurry, adding salt to lower the freezing point below zero degrees Celsius, and then using this mixture to freeze pure water in a test tube. The video also explains the concept of colligative properties and their applications, such as de-icing roads and car radiators, and making ice cream.
Takeaways
- 🧪 The experiment demonstrates freezing water without a freezer by leveraging the concepts of freezing point depression and phase changes.
- 🌡 To start, room temperature water is mixed with crushed ice to create an ice-water mixture, which initially measures at 0°C.
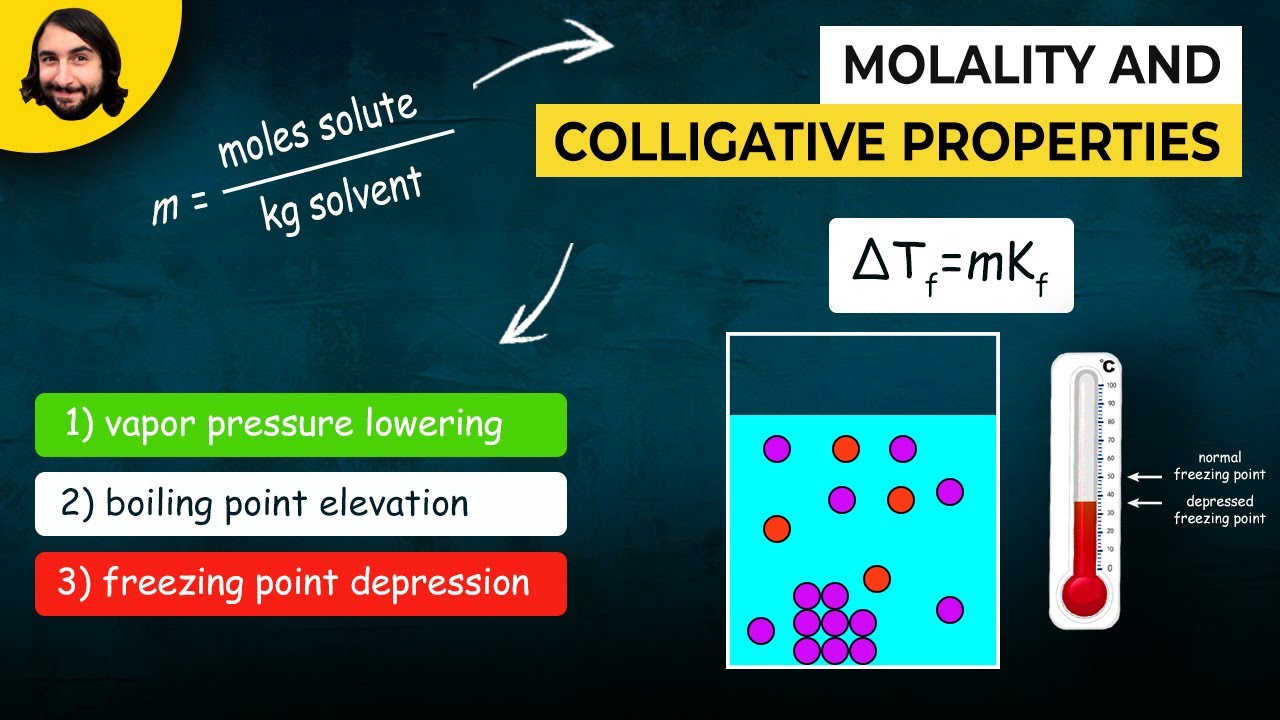
- ❄️ Freezing point depression is achieved by adding salt to the ice-water mixture, which lowers the temperature below 0°C.
- 🧂 The addition of salt disrupts the formation of hydrogen bonds in water, preventing it from freezing until a lower temperature is reached.
- 🕒 The experiment uses a test tube with pure water to show how the salt-depressed ice-water mixture can freeze water in a short time.
- ⏱️ A stopwatch is used to time how long it takes for the water in the test tube to freeze when placed in the cold saltwater bath.
- 🔍 The experiment visually shows the phase change from liquid to solid as the water in the test tube freezes within minutes.
- 🌟 Colligative properties, such as freezing point depression and boiling point elevation, depend on the number of solute particles, not the type of solute.
- 🛣️ Real-world applications of freezing point depression include using salt on roads to prevent ice formation and adding antifreeze to car radiators.
- 🍨 An interesting application mentioned is the use of freezing point depression in the making of chocolate ice cream to achieve a desirable texture.
- 📚 The video script provides a link to a detailed write-up of the experiment for those seeking more information.
Q & A
What are the two main physics topics discussed in the video?
-The two main physics topics discussed in the video are freezing point depression and phase changes.
What is the goal of the experiment presented in the video?
-The goal of the experiment is to freeze water without using a freezer by utilizing the principles of freezing point depression.
What materials are needed for the experiment as mentioned in the video?
-The materials needed for the experiment include a thermometer, salt, ice, crushed ice, a test tube, a stopwatch, water, and beakers or glasses.
How does the addition of salt affect the freezing point of water?
-Adding salt to water causes the freezing point to decrease because the salt ions disrupt the formation of hydrogen bonds between water molecules, thus requiring a lower temperature for the water to freeze.
What is the initial temperature of the ice-water mixture in the experiment?
-The initial temperature of the ice-water mixture is at 0 degrees Celsius, which is the freezing point of pure water.
How does the video demonstrate the concept of freezing point depression?
-The video demonstrates freezing point depression by adding salt to an ice-water mixture, causing the temperature to drop below 0 degrees Celsius, which is the normal freezing point of water.
What is the role of stirring in the experiment?
-Stirring the mixture in the experiment ensures that the salt is evenly distributed and helps the temperature to decrease uniformly, showcasing the effect of freezing point depression.
How long does it take for the water in the test tube to freeze when placed in the saltwater mixture?
-It takes approximately four minutes for the water in the test tube to freeze when placed in the saltwater mixture, which is colder than 0 degrees Celsius.
What is a colligative property and how does it relate to the experiment?
-A colligative property is a physical change that occurs when a solute is added to a solvent, and it depends on the number of solute particles rather than the type of solute. In the experiment, adding salt (solute) to water (solvent) demonstrates the colligative property of freezing point depression.
What are some real-world applications of freezing point depression mentioned in the video?
-Some real-world applications of freezing point depression include using salt on roads to prevent ice formation during winter, using antifreeze in car radiators to prevent the engine's water from freezing, and in the process of making chocolate ice cream.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantVoir Plus de Vidéos Connexes
5.0 / 5 (0 votes)