AP Chem Video 1.2 Measurement, metric system, and conversions
Summary
TLDRThis educational video script delves into the fundamentals of the International System of Units (SI), focusing on base units and the significance of metric prefixes. It explains scientific notation for handling large and small numbers, emphasizing the importance of exponents. The script introduces common metric prefixes and their practical applications in chemistry, providing examples to illustrate the appropriate use of units for different measurements. It also guides viewers through the process of unit conversion using dimensional analysis, with step-by-step examples that demonstrate converting between grams and kilograms, meters and centimeters, and picometers to millimeters.
Takeaways
- 🔍 The script discusses the International System of Units (SI) and focuses on measurement, emphasizing the importance of base units and metric prefixes.
- 📏 Base units in the metric system are those without prefixes, such as meters for length, grams for mass, and seconds for time.
- 🔢 Scientific notation is introduced as a method to write very large or very small numbers, using positive exponents for large numbers and negative exponents for small ones.
- 🌐 Examples given include writing seven billion people as 7 × 10^9 and the diameter of an atom as 1 × 10^-10 meters.
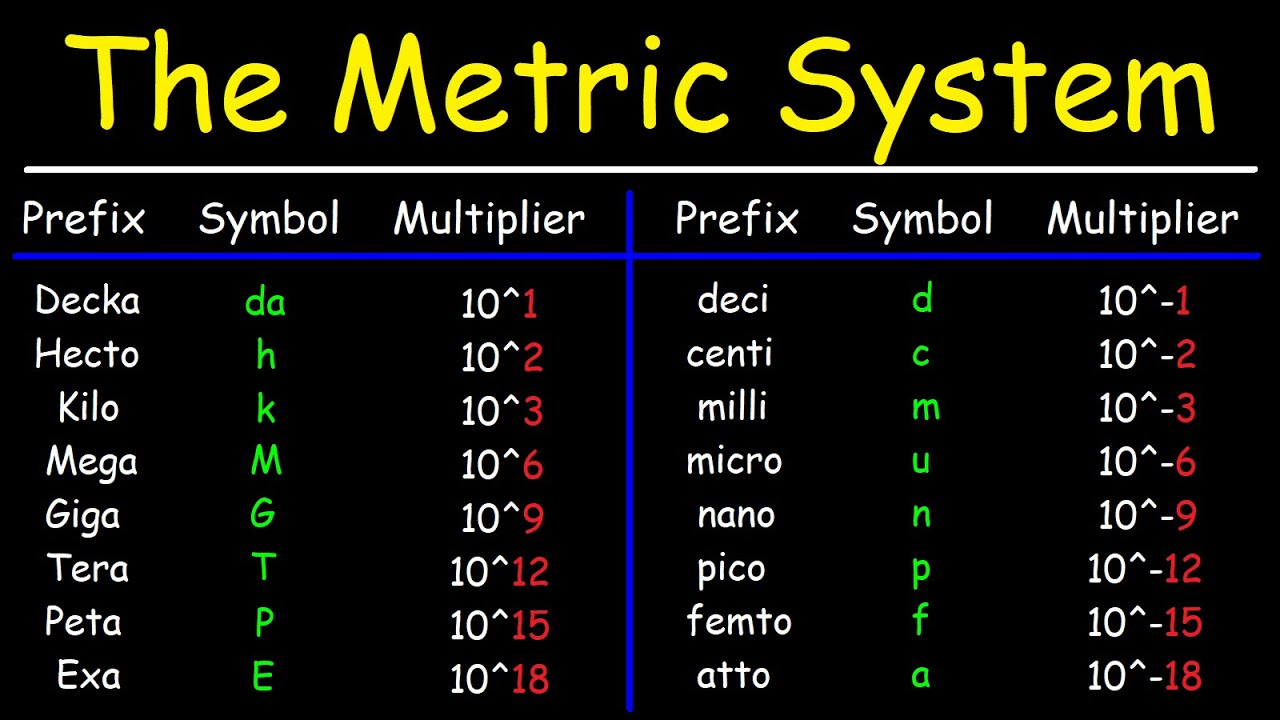
- 📋 The script provides a list of metric prefixes, highlighting the most common ones used in chemistry, such as kilo, centi, milli, micro, and nano.
- 📏 The prefix 'kilo' means 10^3 (thousand), and a kilometer is used as an example of a unit that represents a large distance.
- 📏 The prefix 'centi' means 10^-2 (hundredth), and a centimeter is described as approximately the width of a finger.
- 📏 'Milli' and 'micro' are explained with examples like a millimeter being the thickness of a dime and a red blood cell being about 10 micrometers.
- 🔬 The script explains how to choose appropriate units for different measurements, such as using kilograms for the mass of an airplane and milligrams for the mass of an ant.
- 🔄 The process of dimensional analysis is introduced for unit conversions, demonstrating how to convert between grams and kilograms, and from picometers to millimeters.
- 📘 The script concludes with an example problem that involves multiple steps of conversion, illustrating the use of scientific notation and unit cancellation in calculations.
Q & A
What are base units in the metric system?
-Base units in the metric system are units that do not have prefixes. They are used as the fundamental units of measurement for quantities such as mass, length, and time.
Why is scientific notation important in measurement?
-Scientific notation is important in measurement because it provides a convenient way to express both very large and very small numbers, making it easier to write and understand quantities that would otherwise require many zeros.
How is the number of people in the world expressed in scientific notation?
-The number of people in the world, which is around seven billion, is expressed in scientific notation as 7 x 10^9 people.
What is the difference between large and small numbers in scientific notation?
-Large numbers in scientific notation have positive exponents, while small numbers have negative exponents. This reflects the scale of the number being represented.
What is the diameter of an atom in scientific notation?
-The diameter of an atom is approximately 1 x 10^-10 meters, which is a very small number, hence the negative exponent.
Why do we use metric prefixes?
-We use metric prefixes to correlate with the size of what we are measuring, allowing us to express quantities in a more manageable form without having to write out many zeros.
What is the most common prefix used for measuring the distance from North High School to Fargodome?
-The most common prefix used for measuring the distance from North High School to Fargodome is 'kilo', as a kilometer is approximately the distance between these two places.
How many micrometers is a red blood cell in diameter?
-A red blood cell is about 10 micrometers in diameter from edge to edge.
What is the relationship between a nanometer and a micrometer?
-A nanometer is a thousand times smaller than a micrometer, with one nanometer being 10^-9 meters and one micrometer being 10^-6 meters.
How can you determine the most reasonable unit of measure for an object?
-To determine the most reasonable unit of measure for an object, consider the size of the object relative to common objects and the metric prefixes that correspond to that scale.
What is dimensional analysis and how is it used in conversions?
-Dimensional analysis is a process used in conversions where you set up ratios of equivalent units to change a measurement from one unit to another. It involves using conversion factors to cancel out the original unit and replace it with the desired unit.
How many centimeters are in a meter?
-There are 100 centimeters in a meter, which is represented as 1 meter = 100 centimeters.
How do you convert from Pico meters to millimeters?
-To convert from Pico meters to millimeters, you first convert Pico meters to meters, knowing that there are 10^12 Pico meters in a meter, and then convert meters to millimeters, knowing that there are 1000 millimeters in a meter.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenant5.0 / 5 (0 votes)