Carbon: The Element of Life
Summary
TLDRProfessor Dave explores carbon's unique properties as the 'element of life.' Carbon's ability to form four bonds and its versatile bonding shapes with various elements, including itself, is highlighted. Its role in creating diverse structures like graphite, diamond, and biological molecules is discussed. The video also touches on carbon's applications in materials like steel and Teflon, and its potential in drug synthesis, emphasizing its importance in both natural and synthetic realms.
Takeaways
- 🌌 Carbon is an element with six protons and is crucial for life, being found in all living organisms.
- 🔬 The number of protons defines an element, and carbon's six protons make it unique for its bonding capabilities.
- 🌟 Carbon is formed in stars through nuclear fusion, with heavier elements requiring supernovae or particle accelerators for creation.
- ⚛️ Carbon atoms have four valence electrons, which allows them to form up to four bonds with other atoms.
- 🔗 Carbon's ability to form single, double, or triple bonds with a variety of elements contributes to its versatility.
- 🌐 The geometry of carbon bonding leads to three-dimensional shapes when carbon atoms are bonded to four other atoms.
- 💪 Carbon-carbon bonds are strong yet flexible, allowing for the rearrangement needed for life's complex molecules.
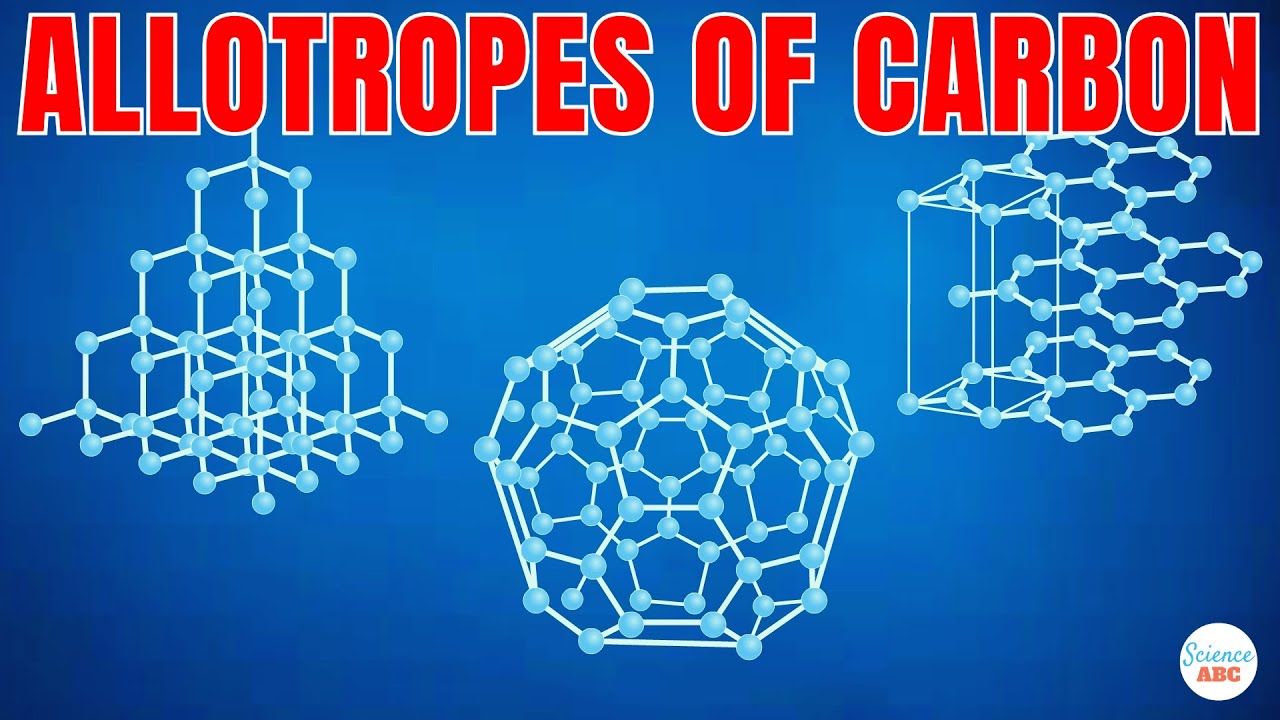
- ⚙️ Carbon exists in various allotropes, including graphite, diamond, nanotubes, and fullerenes, each with unique properties.
- 🧬 Combined with hydrogen, oxygen, nitrogen, and other elements, carbon forms the building blocks of life, such as carbohydrates, proteins, and DNA.
- 🔧 Carbon's properties are harnessed in synthetic materials like steel, Teflon, and carbon-based drugs, showcasing its importance in technology and medicine.
Q & A
What defines an element and how is carbon defined?
-An element is defined by the number of protons in its nucleus. Carbon is defined as an element with six protons.
How are elements like carbon and iron formed in stars?
-Elements such as carbon and iron are formed in stars through nuclear fusion, where protons and neutrons smash together and fuse under tremendous inward pressure.
What role do supernova explosions play in the formation of larger elements?
-Supernova explosions provide the immense energy required for the formation of much larger elements that cannot be formed through normal stellar nuclear fusion.
Why are carbon's valence electrons significant for its chemical properties?
-Carbon's valence electrons are significant because they are available for bonding with other atoms, allowing carbon to form a wide variety of compounds.
How does carbon's ability to form four bonds affect its versatility in bonding?
-Carbon's ability to form four bonds makes it versatile as it can create single, double, or triple bonds with many different elements, contributing to its importance in forming complex molecules.
What is unique about the three-dimensional shape that carbon atoms adopt when bonded to four other atoms?
-When bonded to four other atoms, carbon atoms adopt a three-dimensional shape to maximize the distance between electron clouds, which is unique and allows for complex molecular structures.
How do the properties of carbon-carbon bonds contribute to carbon's role as a building block in various materials?
-Carbon-carbon bonds are strong enough to be stable but not so strong that they can't break and rearrange, making them excellent building blocks for a wide range of materials.
What are some of the allotropes of carbon mentioned in the script?
-The script mentions graphite, diamond, nanotubes, and fullerenes as some of the allotropes of carbon.
How does carbon combine with hydrogen, oxygen, and nitrogen to form biologically significant molecules?
-Carbon combines with hydrogen, oxygen, and nitrogen, along with a few other elements, to form diverse structures in the body, including carbohydrates, proteins, and DNA.
What is an example of how carbon is used to enhance the properties of other materials?
-Carbon is used to enhance the properties of iron by placing it in the empty spots in an iron lattice, resulting in steel, which is stronger and used in most large structures.
How do carbon-based synthetic drugs contribute to medical advancements?
-Carbon-based synthetic drugs, such as inhibitors that can silence faulty enzymes, hold the prospect of curing many diseases, showcasing the importance of carbon in medical science.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantVoir Plus de Vidéos Connexes

The Structural Characteristics of CARBON | Carbon Compounds | Grade 9 Science Quarter 2 Week 4-5

Propriedades da Água (Componentes químicos dos seres) - Aula 2 - Mód 1 - Bioquímica - Prof Guilherme

Understanding Vector Spaces
Allotropes of Carbon Explained in Simple Words for Beginners

Why is All Life Carbon Based, Not Silicon? Three Startling Reasons!

Properties of Carbon
5.0 / 5 (0 votes)
