21.2-Carboxylic Acid Derivatives
Summary
TLDRThis video explores carboxilic acid derivatives, discussing their reactivity, naming conventions, and how they differ from carboxilic acids. It delves into the stability of leaving groups, the role of resonance in reactivity, and the mechanisms of interconversion between derivatives, emphasizing the importance of nucleophilic attack and leaving group steps in reactions.
Takeaways
- 🌟 Carboxilic acid derivatives are compounds where the OH group of a carboxylic acid is replaced with other functional groups containing oxygen, such as O, NR2, or Cl.
- 🔍 Different types of carboxylic acid derivatives include acid chlorides, anhydrides, esters, amides, and nitriles, each with varying reactivities.
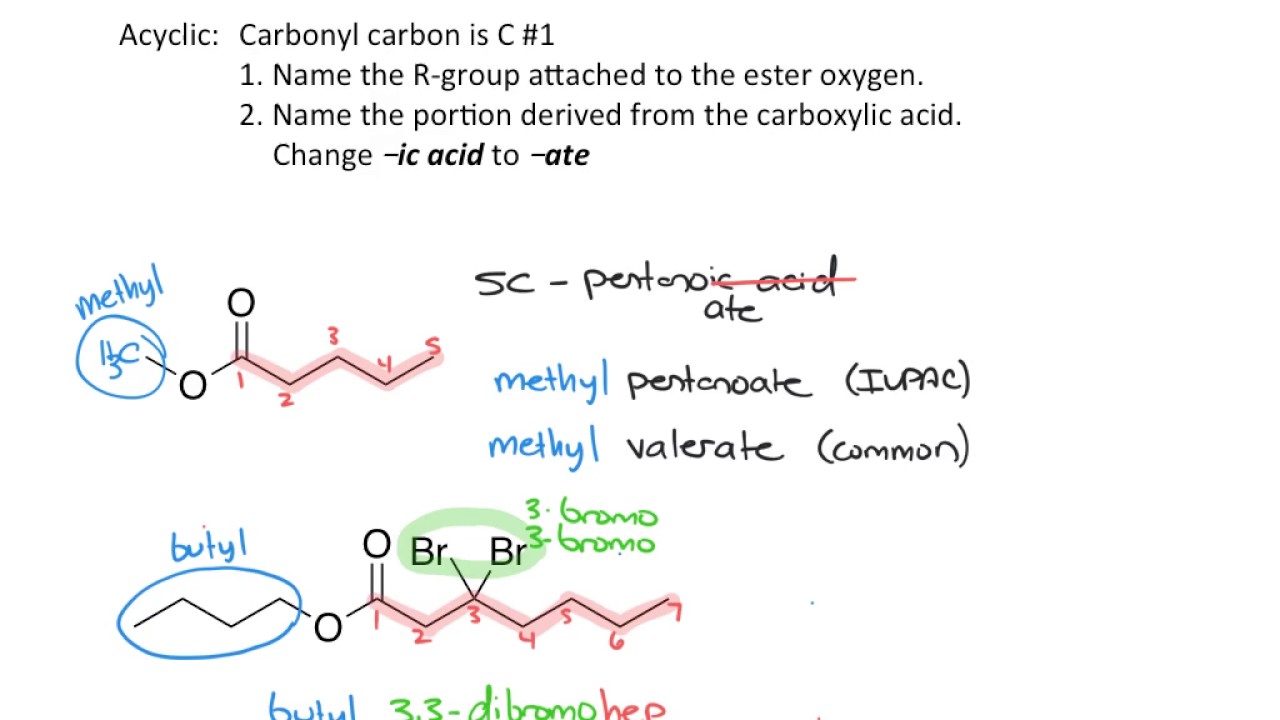
- 📚 Naming conventions for these derivatives involve removing 'acid' for acid chlorides, adding 'ide' for anhydrides, and using alkyl groups for esters and amides.
- 💡 The reactivity of carboxylic acid derivatives can be understood through the stability of the leaving group, the electronegativity of the attached atom, and resonance structures.
- 🌡 Acid chlorides are the most reactive due to the stability of the chloride leaving group, while amides are the least reactive due to the least electrophilic carbon.
- 🔄 The interconversion of carboxylic acid derivatives typically involves nucleophilic attack, followed by the reformation of a carbon-oxygen double bond and a leaving group.
- 🚫 Under acidic conditions, avoid generating negatively charged intermediates, and under basic conditions, avoid positively charged intermediates.
- 🌐 Resonance structures play a significant role in determining reactivity, with the more positive charge character on carbon indicating higher reactivity.
- 💧 Hydrolysis of esters is an example of a reaction involving carboxylic acid derivatives, where water acts as a nucleophile under both acidic and basic conditions.
- 📉 The reactivity order of carboxylic acid derivatives from most to least reactive is: acid chlorides > anhydrides > esters > amides.
Q & A
What are carboxilic acid derivatives?
-Carboxilic acid derivatives are compounds that result from modifying the hydroxyl group (-OH) of a carboxilic acid. They differ in reactivity due to the change in the nature of the group attached to the carbonyl carbon, which can be an O, NR2, or Cl.
What is an acid chloride and how is it named?
-An acid chloride is a type of carboxilic acid derivative where the hydroxyl group is replaced by a chlorine atom. It is named by replacing the word 'acid' in the carboxilic acid name with 'chloride', for example, benzoic acid chloride is named benzo chloride.
What is an anhydride and how does it differ from other carboxilic acid derivatives?
-An anhydride is a carboxilic acid derivative where the hydroxyl group is replaced by an oxygen atom that forms a double bond with another carbon. It differs from other derivatives in that it contains a carbon-oxygen double bond, unlike esters, amides, or nitriles.
How are esters named in the context of carboxilic acid derivatives?
-Esters are named as alkyl alkanoates, where 'alkyl' refers to the alkyl group attached to the carboxilic acid and 'alkanoate' is derived from the carboxilic acid name, for instance, ethyl acetate from acetic acid.
What is an amide and how does its naming differ from other derivatives?
-An amide is a carboxilic acid derivative with a nitrogen containing group, where the hydroxyl group is replaced by an NR2 group. It is named by dropping the 'oic' from the carboxilic acid name and adding 'amide', such as propionic acid becoming propenamide.
How does the stability of the leaving group affect the reactivity of carboxilic acid derivatives?
-The reactivity of carboxilic acid derivatives is influenced by the stability of the leaving group. A more stable leaving group, such as chloride, results in a more reactive derivative because it can more readily depart, leaving behind a nucleophile attached to the electrophilic carbon.
What role does resonance play in determining the reactivity of carboxilic acid derivatives?
-Resonance significantly affects the reactivity by distributing the charge across the molecule. In derivatives like amides, the resonance structure contributes more to the hybrid, stabilizing the positive charge on carbon and making the amide less reactive compared to other derivatives like acid chlorides.
How can one convert an acid chloride to an ester?
-An acid chloride can be converted to an ester through nucleophilic attack by an alcohol, followed by the departure of the chloride ion as the leaving group, resulting in the formation of a new ester and a chloride ion.
What is a nucleophilic attack and why is it important in the conversion of carboxilic acid derivatives?
-Nucleophilic attack is a chemical reaction where a nucleophile, a species with a lone pair of electrons, donates its electrons to an electrophilic center, typically a carbon atom in carboxilic acid derivatives. It is important because it initiates the conversion process from one derivative to another.
Why can't carbon or hydrogen act as leaving groups in the reactions of carboxilic acid derivatives?
-Carbon and hydrogen cannot act as leaving groups in reactions of carboxilic acid derivatives because they are less electronegative than nitrogen, oxygen, or chlorine and cannot stabilize a negative charge. Thus, they are too unstable to leave the molecule.
How do proton transfer steps affect the mechanism of reactions involving carboxilic acid derivatives?
-Proton transfer steps can influence the mechanism by stabilizing intermediates under certain conditions. For instance, under acidic conditions, a proton can be transferred to an oxygen to make it a better leaving group, facilitating the reaction progress.
What is the general rule for writing mechanisms involving carboxilic acid derivatives?
-The general rule for writing mechanisms involving carboxilic acid derivatives is to include nucleophilic attack and leaving group departure in every mechanism. Additionally, avoid generating negative charges under acidic conditions and positive charges under basic conditions.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahora5.0 / 5 (0 votes)