Peripheral T cell Tolerance - Clonal Deletion and Anergy (FL-Immuno/78)
Summary
TLDRThe lecture discusses peripheral T-cell tolerance, explaining how autoreactive T cells, which escape central tolerance, are managed to prevent autoimmune responses. Key mechanisms include clonal deletion, where autoreactive T cells are eliminated through apoptosis, and anergy, which inactivates T cells without complete activation. The role of dendritic cells is highlighted, as they present self-antigens in the absence of danger signals, leading to T cell tolerance. Additional mechanisms like immune deviation and regulatory T cells further control immune responses. The importance of maintaining immune homeostasis is emphasized, setting the stage for a subsequent discussion on B-cell tolerance.
Takeaways
- 😀 Central T-cell tolerance eliminates auto-reactive T cells in early developmental stages within central lymphoid organs, but it is not foolproof.
- 😀 Peripheral tolerance prevents self-reactive T cells from attacking tissues, with mechanisms like clonal deletion, anergy, immune deviation, and regulatory T cells.
- 😀 Danger signals such as cytokines, chemokines, and microbial products are produced during infections or injuries, activating dendritic cells and triggering immune responses.
- 😀 Immature dendritic cells mature in response to cytokines, and once activated, they present antigens to naive T cells in lymphoid tissues.
- 😀 Complete activation of naive T cells requires three signals: recognition of antigen-MHC complex, co-stimulation (via B7-CD28 interaction), and cytokine release from T cells.
- 😀 Autoreactive T cells recognize self-antigens, which are not associated with danger signals, making them different from responses to infections or injuries.
- 😀 In the absence of danger signals, dendritic cells do not express co-stimulatory molecules, leading to the deletion or inactivation of autoreactive T cells.
- 😀 Peripheral clonal deletion occurs when autoreactive T cells are eliminated by apoptosis, while anergy occurs when these cells are inactivated without differentiation into effector cells.
- 😀 Immune deviation shifts harmful immune responses to less harmful ones, while immune privilege refers to body sites less exposed to immune responses (e.g., CNS, eyes, testes).
- 😀 Immunosuppressive molecules like IL-10 and TGF-beta inhibit immune responses by suppressing macrophages, inflammatory cytokines, and antigen-presenting cells.
- 😀 Regulatory T cells control activated T cell responses, block proliferation, and secrete immunosuppressive cytokines, contributing to peripheral tolerance.
Q & A
What is the main purpose of peripheral tolerance?
-The main purpose of peripheral tolerance is to ensure that autoreactive T cells that escaped central tolerance do not attack self tissues, preventing autoimmune responses.
What are the key mechanisms of peripheral tolerance?
-The key mechanisms of peripheral tolerance include peripheral clonal deletion, anergy, immune deviation, immune privilege, immunosuppressive molecules, and regulatory T cells (Tregs).
What is peripheral clonal deletion, and how does it work?
-Peripheral clonal deletion is a process where autoreactive T cells are eliminated through programmed cell death (apoptosis) when they bind to self-antigens presented by dendritic cells without co-stimulatory signals.
What role do dendritic cells play in peripheral tolerance?
-Dendritic cells process and present self-antigens to T cells. In the absence of danger signals, they do not express co-stimulatory molecules, leading to T cell apoptosis or anergy.
What happens when a T cell binds to a self-antigen without co-stimulation?
-When a T cell binds to a self-antigen without co-stimulation, it undergoes apoptosis or becomes anergic, preventing it from differentiating into effector cells and avoiding an immune response.
How does immune deviation contribute to peripheral tolerance?
-Immune deviation refers to the alteration of a potentially harmful immune response to a less harmful one, reducing the risk of autoimmune damage and maintaining tolerance.
What is the significance of immune privilege in peripheral tolerance?
-Immune privilege refers to anatomical sites like the brain, eyes, and testes, which are less susceptible to immune responses, thereby reducing the risk of self-reactive immune activity in these areas.
How do immunosuppressive molecules contribute to peripheral tolerance?
-Immunosuppressive molecules like IL-10 and TGF-beta inhibit macrophage activation, reduce inflammatory cytokine secretion, downregulate T cell signaling, and block antigen-presenting cell function, helping control the immune response.
What is the function of regulatory T cells (Tregs) in peripheral tolerance?
-Regulatory T cells (Tregs) control the responses of activated T cells by blocking their proliferation and secreting immunosuppressive cytokines, maintaining immune tolerance.
What happens when dendritic cells present self-antigens without danger signals?
-When dendritic cells present self-antigens without danger signals, they do not express co-stimulatory molecules, leading to the absence of second signals required for T cell activation. This results in the apoptosis or anergy of autoreactive T cells.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados

Immune tolerance - An introduction (FL-Immuno/76)

Roles of Regulatory T Cells | Immunology | Immune System | Basic Science Series

Hipersensitivitas Tipe 4 (Cell-mediated Hypersensitivity), Immunology
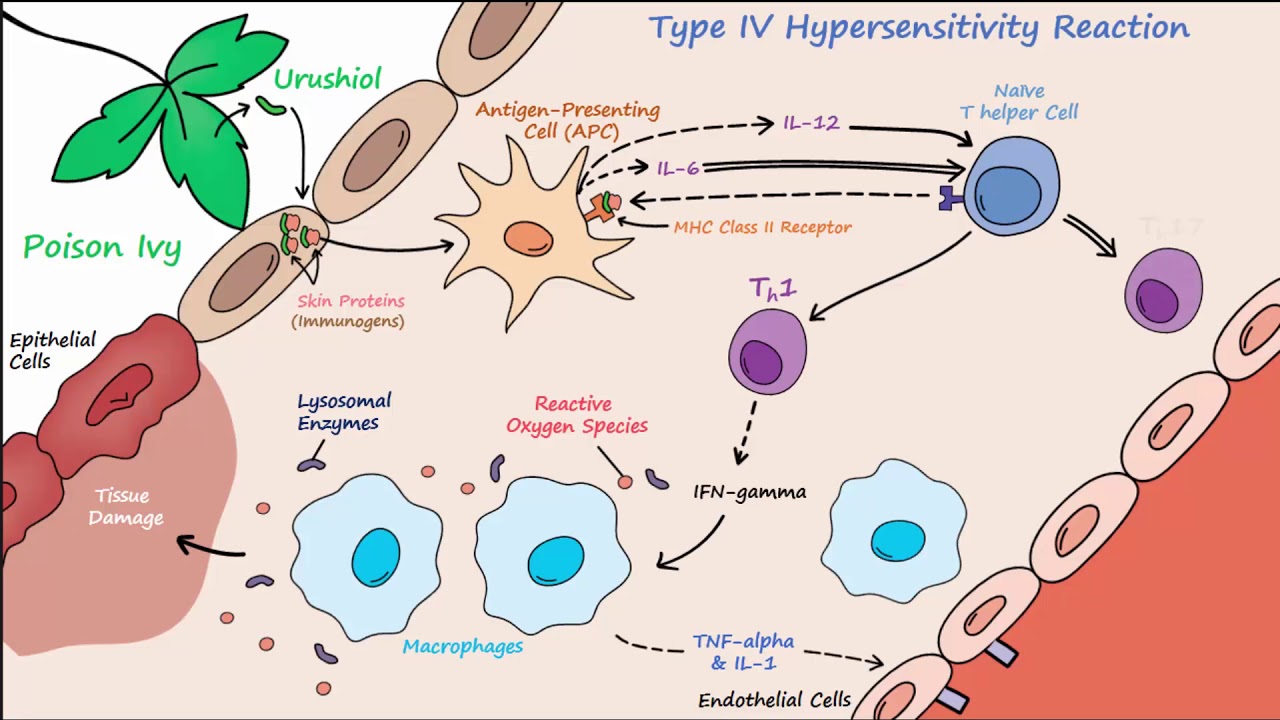
Type IV Hypersensitivity (Described Concisely)
Immunology Lecture 8 (T Cells Maturation and Selection) 1/3

Immunology Thymus Tutorial
5.0 / 5 (0 votes)
