SIFAT KOLIGATIF LARUTAN : PENURUNAN TITIK BEKU
Summary
TLDRThe video explores colligative properties, focusing on freezing point depression. It explains how adding solutes, like salt or ethylene glycol, lowers the freezing point of water, preventing ice formation in cold climates. Through practical experiments, it demonstrates that solutions with solutes freeze at lower temperatures than pure solvents. The video also discusses how the number of particles from electrolytes affects freezing points, with stronger electrolytes leading to greater depression. It concludes by providing mathematical equations for calculating freezing point depression, emphasizing the significance of understanding these properties in real-world applications.
Takeaways
- 😀 The video discusses the colligative properties of solutions, focusing on freezing point depression.
- 😀 In cold countries, salt (like CaCl2) is spread on roads to melt snow and improve safety.
- 😀 Adding solutes like sugar to water lowers its freezing point compared to pure water.
- 😀 The freezing point of water is 0°C at 760 mm Hg, but adding solutes changes this equilibrium.
- 😀 A demonstration is suggested using ice, salt, and sugar solutions to observe differences in freezing points.
- 😀 Freezing point depression occurs because solutes hinder the formation of solid phases from liquids.
- 😀 The relationship between freezing point depression (ΔTF) and concentration of solutes can be mathematically expressed.
- 😀 Electrolytes can further decrease freezing points compared to non-electrolytes due to ion dissociation.
- 😀 The Van 't Hoff factor (i) is essential for calculating freezing point depression in electrolyte solutions.
- 😀 Example calculations demonstrate how to determine the freezing points for various solutes, including ethylene glycol and CaCl2.
Q & A
What are colligative properties?
-Colligative properties are properties of solutions that depend on the number of solute particles in a given amount of solvent, rather than the identity of the solute. Examples include vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.
How does the addition of salt affect the freezing point of water?
-The addition of salt lowers the freezing point of water, meaning that a saltwater solution will freeze at a lower temperature than pure water. This is due to the interference of solute particles with the formation of solid ice.
What is the significance of the term 'freezing point'?
-The freezing point is the temperature at which a liquid becomes a solid. It is defined as the temperature at which the liquid and solid phases coexist in equilibrium under a specific pressure, typically 760 mm Hg.
What happens when a non-electrolyte solute, like sugar, is added to water?
-When a non-electrolyte solute such as sugar is added to water, it lowers the freezing point of the solution compared to pure water. The more solute added, the lower the freezing point becomes.
How can the freezing point depression be mathematically calculated?
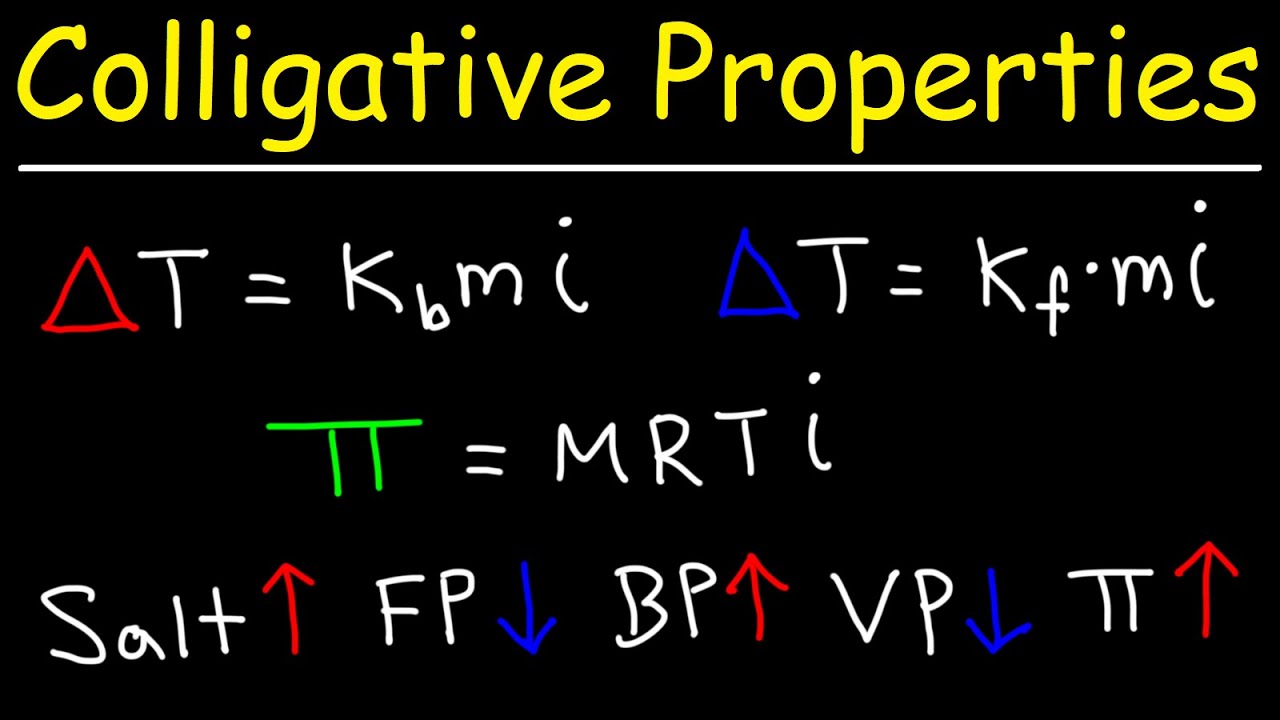
-Freezing point depression can be calculated using the formula: ΔTf = Kf * m, where ΔTf is the depression in freezing point, Kf is the cryoscopic constant, and m is the molality of the solution.
What is the role of ethylene glycol in automotive radiators?
-Ethylene glycol is added to automotive radiators to lower the freezing point of the coolant. This prevents the coolant from freezing at low temperatures, ensuring the engine operates efficiently.
What is the effect of strong electrolytes on the freezing point of solutions?
-Strong electrolytes, which dissociate completely in solution, produce more solute particles compared to non-electrolytes or weak electrolytes. This leads to a greater freezing point depression.
How is the Van 't Hoff factor related to freezing point depression?
-The Van 't Hoff factor (i) indicates the number of particles a solute produces in solution. For electrolytes, this factor is essential in calculating freezing point depression because it accounts for the degree of ionization.
What happens to the freezing point of a solution as the concentration of solute increases?
-As the concentration of solute increases, the freezing point of the solution decreases further. This means a higher concentration of solute requires a lower temperature to freeze.
How do you determine the degree of ionization of a weak electrolyte from freezing point data?
-To determine the degree of ionization of a weak electrolyte, you can calculate the Van 't Hoff factor from freezing point depression data and use the formula: i = 1 + α(n - 1), where α is the degree of ionization and n is the number of ions produced.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados

Penurunan Titik Beku Larutan

13.2 Colligative Properties of Solutions (1/2)
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure

SKL (2)| Kenaikan Titik Didih (∆Tb) | Penurunan Titik Beku (∆Tf)

Diagram fase air dan larutan NaOH adalah sebagai A B berikut, P Keterangan: 6 perubahan fase air ...

Química Simples #12 - Resumos - Propriedades Coligativas
5.0 / 5 (0 votes)
