Quantum Mechanics - Part 2: Crash Course Physics #44
Summary
TLDRThis video explores the intriguing concept of wave-particle duality, initially proposed by Louis de Broglie, suggesting that all matter, like light, behaves as both a particle and a wave. Through the double-slit experiment, it illustrates how electrons create interference patterns, confirming de Broglie's hypothesis. The discussion includes Schrödinger's wavefunction and its role in predicting particle behavior through probability density functions. Additionally, it highlights the Heisenberg Uncertainty Principle, emphasizing the limits of measuring position and momentum simultaneously. Overall, the video invites viewers into the strange and counterintuitive world of quantum mechanics.
Takeaways
- 🌌 Wave-particle duality suggests that light and all matter can exhibit both particle-like and wave-like behaviors.
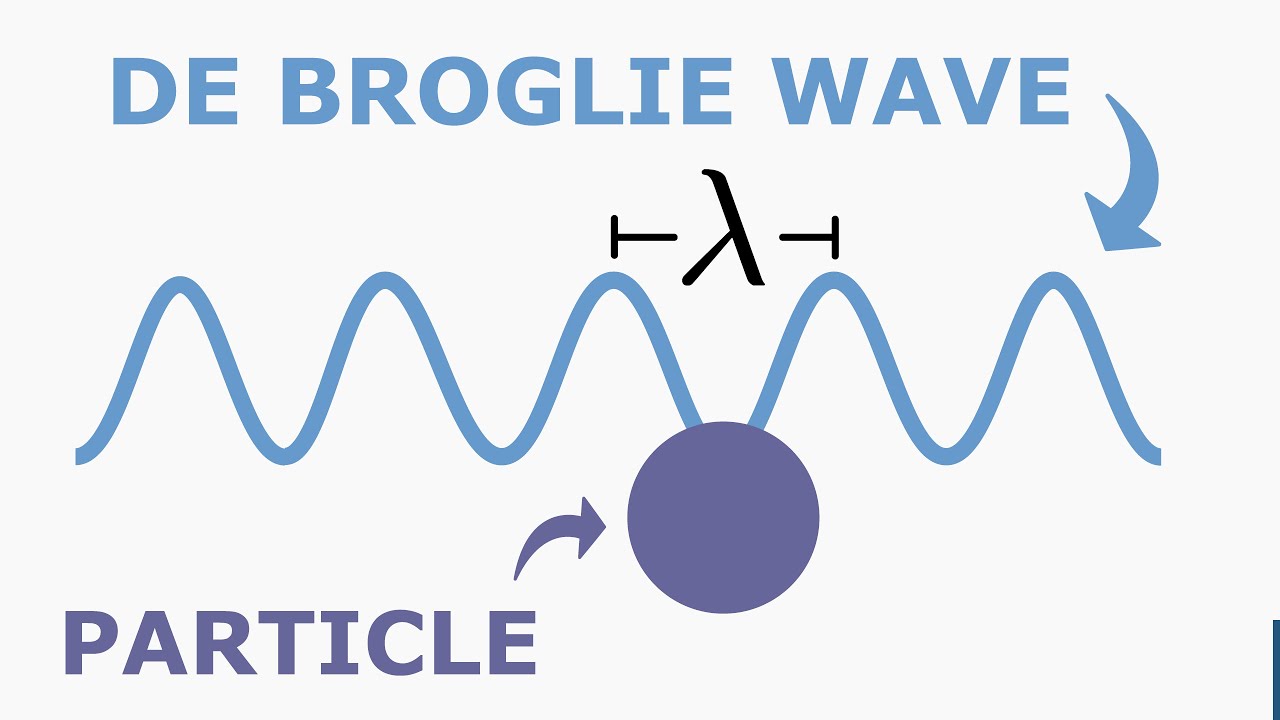
- 🔬 Louis de Broglie proposed that all matter has an associated wave, extending the wave-particle duality concept beyond light.
- ⚛️ The double-slit experiment demonstrated that electrons create interference patterns, confirming their wave-like nature.
- 📏 The wavelength of an object is determined by its momentum and is calculated using Planck's constant, leading to incredibly small wavelengths for large objects.
- 🔍 Quantum mechanics heavily relies on probabilities, particularly in predicting the behavior of particles like electrons.
- 🧮 Schrödinger's equation describes how the quantum state of a system evolves over time and helps determine probability density functions.
- 🔄 The probability density function visualizes the likelihood of finding a particle in various locations around a nucleus.
- 🐱 Schrödinger's thought experiment with a cat illustrates the counterintuitive concept of quantum superposition, where a particle can exist in multiple states until observed.
- 📉 The Heisenberg Uncertainty Principle states that the more precisely we measure one property (position or momentum) of a particle, the less precisely we can measure the other.
- 🤯 Quantum mechanics challenges our classical understanding of reality, emphasizing the strange and probabilistic nature of the universe.
Q & A
What is wave-particle duality?
-Wave-particle duality is the concept in physics that light and matter can exhibit both wave-like and particle-like properties. This means that entities like photons (light particles) and electrons can behave as both waves and particles depending on the experiment.
Who proposed the idea that all matter has an associated wave?
-The idea that all matter has a wave associated with it was proposed by French physicist Louis de Broglie in 1923.
What was the significance of de Broglie's proposal?
-De Broglie's proposal was significant because it extended the wave-particle duality concept beyond light to all matter, leading to a deeper understanding of quantum mechanics and the behavior of tiny particles.
How did the double slit experiment demonstrate wave behavior in electrons?
-In the double slit experiment, a beam of electrons was shot through two slits, producing a diffraction pattern on a screen behind the slits. This pattern, similar to that created by light waves, indicated that electrons can behave like waves and interfere with each other.
Why don't we observe the wave nature of larger objects?
-We don't observe the wave nature of larger objects because their wavelengths, calculated using Planck's constant and momentum, are extremely small. For example, a baseball's wavelength is so tiny that it's impossible to detect with the naked eye.
What role does probability play in quantum mechanics?
-Probability is fundamental in quantum mechanics, particularly in predicting the behavior of particles. The wavefunction represents the probability density function, indicating the likelihood of finding a particle at specific locations.
What is Schrödinger's equation, and why is it important?
-Schrödinger's equation is a fundamental equation in quantum mechanics that describes how the quantum state of a physical system changes over time. It is important because it helps calculate the probability density of finding particles in various locations.
What is quantum superposition?
-Quantum superposition is the principle that a quantum system can exist in multiple states at once. For example, in Schrödinger's thought experiment, a cat in a box can be considered both alive and dead until observed.
What does the Heisenberg Uncertainty Principle state?
-The Heisenberg Uncertainty Principle states that it is impossible to precisely know both the position and momentum of a particle at the same time. The more accurately one is known, the less accurately the other can be measured.
How does the wave packet concept relate to measuring particles?
-Wave packets combine multiple waves to describe particles, allowing physicists to estimate the position of a particle while accepting a degree of uncertainty in its momentum. This approach helps reconcile the particle-wave duality in quantum mechanics.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados
De-Broglie Wavelength (Hypothesis) And The Wave-Like Matter

Kuantum 2 .1 Hipotesis de Brogglie (Apakah Partikel Mempunyai sifat Gelombang?)

Por Que Precisamos da Dualidade Onda-Partícula?

Menjawab Misteri Besar Fisika: Apakah Cahaya Gelombang atau Partikel?

vid 2

Davisson-Germer Experiment & Wave-Particle Duality
5.0 / 5 (0 votes)
