What is the Heisenberg Uncertainty Principle? - Chad Orzel
Summary
TLDRThe Heisenberg Uncertainty Principle, a cornerstone of quantum mechanics, posits that it's impossible to know both the exact position and momentum of a particle simultaneously. This principle arises from the dual particle-wave nature of matter, where particles exhibit wave-like properties with wavelength related to momentum. The more precisely the position is measured, the less certain the momentum becomes, and vice versa. This isn't a measurement issue but a fundamental aspect of quantum physics, illustrating the inherent uncertainty in the properties of particles at the subatomic level.
Takeaways
- 🌌 The Heisenberg Uncertainty Principle is a fundamental concept in quantum physics that has permeated popular culture, symbolizing inherent uncertainty.
- 🔍 It asserts that it's impossible to know both the exact position and exact speed (momentum) of a particle simultaneously.
- 🌊 The principle arises because quantum objects exhibit wave-particle duality, behaving as both a particle and a wave.
- 📊 In quantum mechanics, the exact position and momentum of an object are not well-defined until measured.
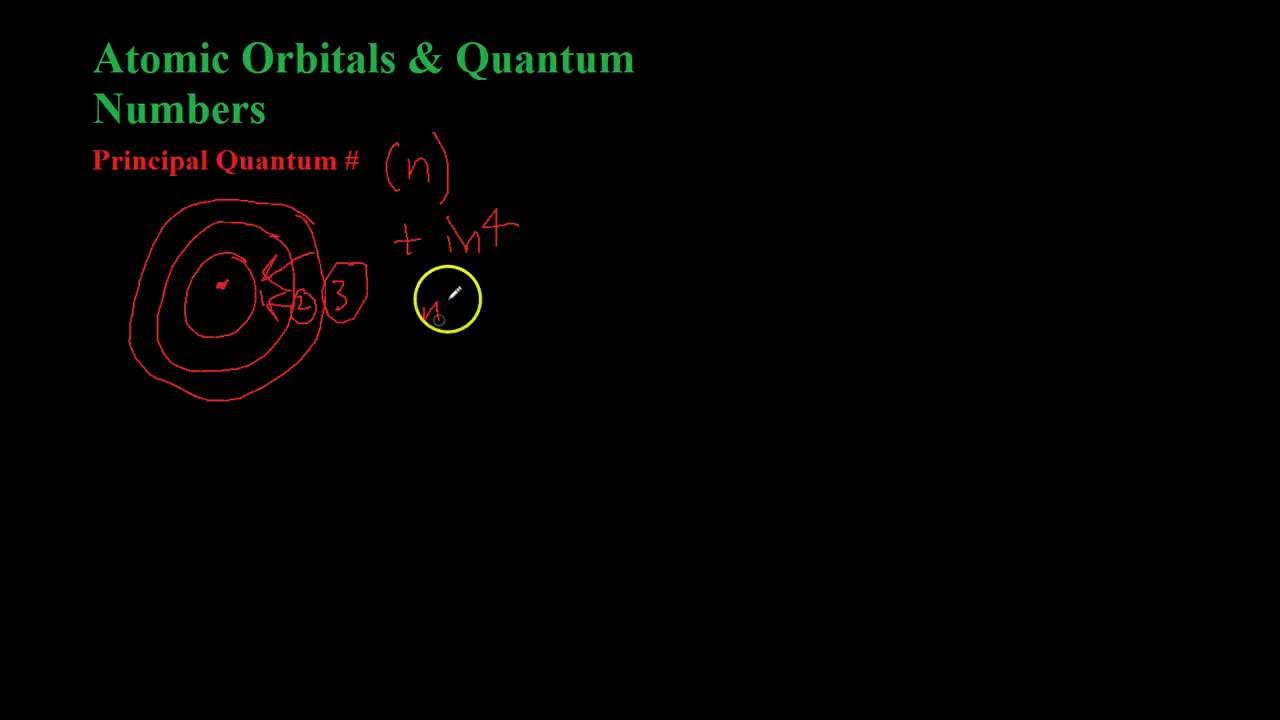
- 🏓 Particles are defined as existing in a single place at any instant, represented by a probability spike at one position.
- 🌀 Waves are spread out in space, with features like wavelength that can't be assigned a single position but have probabilities across many.
- 🔗 The wavelength of an object relates to its momentum, with faster or heavier objects having shorter wavelengths.
- 🏞 Everyday objects don't exhibit wave nature due to their minuscule wavelengths, but atomic or subatomic particles can.
- 🤔 To measure both position and momentum, a wave packet is created by combining waves of different wavelengths, introducing uncertainty.
- 🔄 The act of reducing position uncertainty by creating a smaller wave packet increases momentum uncertainty, and vice versa.
- 📖 Heisenberg's principle isn't about measurement limitations but reflects a fundamental property of the universe, inherent in its structure.
Q & A
What is the Heisenberg Uncertainty Principle?
-The Heisenberg Uncertainty Principle states that it is impossible to simultaneously know the exact position and the exact speed (momentum) of an object, reflecting the inherent uncertainty in the properties of particles at the quantum level.
Why is the Heisenberg Uncertainty Principle significant in popular culture?
-The Heisenberg Uncertainty Principle has become a metaphor for uncertainty in various fields, from literary criticism to sports commentary, because it captures the idea that complete certainty is unattainable, which resonates in many contexts.
How is the uncertainty principle related to the act of measurement?
-The uncertainty principle is often misconceived as a result of measurement. However, the principle is more fundamentally about the nature of quantum objects behaving as both particles and waves, which inherently limits the precision with which their position and momentum can be known.
What does it mean for an object to behave like a particle?
-An object behaving like a particle exists in a single place at any instant in time, which can be represented by a probability graph showing a spike at one specific position and zero elsewhere.
How is the concept of a wave different from that of a particle?
-Waves are disturbances spread out in space, like ripples on a pond, and do not have a single position. They can be characterized by their wavelength, which is the distance between two neighboring peaks or valleys.
Why is wavelength important in quantum physics?
-Wavelength is crucial in quantum physics because it is related to an object's momentum (mass times velocity). A fast-moving or heavy object has a short wavelength, which is significant for understanding the behavior of quantum objects.
Why don't we notice the wave nature of everyday objects?
-The wave nature of everyday objects like a baseball is not noticeable because their wavelengths are extremely small, far beyond detection, due to their mass and speed.
How can small objects like atoms or electrons exhibit measurable wave properties?
-Small objects such as atoms or electrons can have wavelengths that are large enough to be measured in physics experiments, making their wave nature observable.
How does combining waves with different wavelengths affect the position and momentum of a quantum object?
-Combining waves with different wavelengths allows for the creation of a wave packet with a defined position but uncertain momentum, reflecting the trade-off between knowing an object's position and momentum due to the Heisenberg Uncertainty Principle.
What is the relationship between the size of a wave packet and the uncertainty of position and momentum?
-A smaller wave packet, which would localize the position of a quantum object, inherently increases the uncertainty in its momentum, and vice versa. This relationship is a direct consequence of the Heisenberg Uncertainty Principle.
Who first stated the Heisenberg Uncertainty Principle, and when was it stated?
-The Heisenberg Uncertainty Principle was first stated by German physicist Werner Heisenberg in 1927.
Is the uncertainty principle a practical limit on measurement or a fundamental property of the universe?
-The uncertainty principle is not just a practical limit on measurement but a fundamental property of the universe, reflecting the inherent limitations in the properties that quantum objects can possess.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahora5.0 / 5 (0 votes)