Heat Transfer - Conduction, Convection, and Radiation
Summary
TLDRThis educational video script delves into the three primary methods of heat transfer: conduction, convection, and radiation. It explains conduction as the direct heat flow between objects in contact, highlighting the role of materials like metals as conductors and wood or fiberglass as insulators. The script then moves on to convection, describing how fluids, both liquids and gases, transfer heat through movement, using examples like heated water in a beaker and warm air rising on a hot day. Lastly, it covers radiation, the emission of heat through electromagnetic waves across empty space, exemplified by the sun's warming effect on Earth. The video also touches on how objects' colors affect their absorption or reflection of radiation, advising light-colored clothing for summer to stay cool.
Takeaways
- 🔥 Conduction is the transfer of heat through direct contact, where heat flows from a hotter object to a cooler one until thermal equilibrium is reached.
- 🌡️ Thermal equilibrium occurs when two objects in contact have the same temperature, resulting in no net heat flow between them.
- 🛠️ Metals are good conductors of heat, with copper having a high thermal conductivity value of 380 joules per second per meter per Celsius.
- 🏺 Insulators resist the flow of heat, with wood and fiberglass being examples of materials that do not conduct heat well.
- 🔨 The handle of a hot pan is usually made of an insulating material like wood to prevent heat transfer and burns.
- 🌡️ Thermal conductivity is a measure of a material's ability to conduct heat, with values varying widely from conductors to insulators.
- 💨 Convection is the transfer of heat by the movement of fluids, such as water or air, where heated molecules rise and cooler ones sink.
- 🌬️ Air is a good insulator when still, but when moving, it can carry away heat through convection, making us feel cooler or colder.
- 👔 Wearing layers of clothing traps still air, which serves as an excellent insulator, better than the clothes themselves.
- 🌞 Radiation is the transfer of heat through electromagnetic waves across empty space, like the sun's heat reaching Earth.
- 👕 Light-colored clothing reflects more radiation, keeping us cooler in the sun, while dark colors absorb radiation and get hotter.
- ♨️ Every object above absolute zero emits some form of radiation, with the amount increasing as the object's temperature rises.
Q & A
What are the three methods of heat transfer discussed in the script?
-The three methods of heat transfer discussed are conduction, convection, and radiation.
How does heat conduction occur?
-Heat conduction occurs through contact, where heat flows from a region of high temperature to a region of low temperature until thermal equilibrium is reached.
What is thermal equilibrium in the context of heat transfer?
-Thermal equilibrium is when the temperatures of two objects in contact are the same, resulting in no net flow of heat between them.
Why are metals considered good conductors of heat?
-Metals are considered good conductors of heat because they can conduct thermal energy very efficiently, as indicated by their high thermal conductivity values.
What are insulators and why are they used in certain applications?
-Insulators are materials that resist the flow of heat. They are used in applications where it is important to prevent heat transfer, such as in the handle of a hot pan to prevent burns.
What is the difference between thermal conductivity values of copper and water?
-Copper has a thermal conductivity of 380 joules per second per meter per celsius, while water has a much lower value of 0.56, indicating copper is a better conductor of heat.
How does the movement of air affect its insulating properties?
-When air is still, it is a good insulator. However, when it is moving, it can carry away heat through convection, reducing its insulating effectiveness.
What is convection and how does it differ from conduction?
-Convection is the transfer of heat by the movement of a fluid, such as water or air. It differs from conduction, which involves direct contact and does not involve fluid movement.
Why does warm air rise and cold air sink?
-Warm air rises because it is less dense than cold air due to molecular expansion when heated. This results in hot air having less mass per unit volume and therefore rising, while cold air, being denser, sinks.
How does radiation differ from the other two methods of heat transfer?
-Radiation is the transfer of heat through empty space by electromagnetic waves and does not require a medium or contact, unlike conduction and convection.
What role does the color of an object play in its interaction with electromagnetic radiation?
-Light-colored objects reflect more electromagnetic radiation and stay cooler, while dark-colored objects absorb more radiation and get hotter, which is why light-colored clothing is recommended in the summer.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados

Heat Transfer in the Atmosphere - Earth Science for Kids!

Heat Transfer: Conduction, Convection, and Radiation
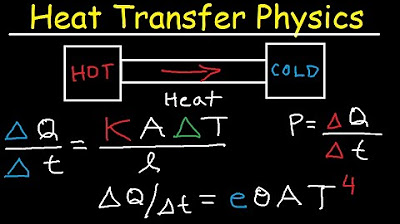
Thermal Conductivity, Stefan Boltzmann Law, Heat Transfer, Conduction, Convecton, Radiation, Physics

Conduction -Convection- Radiation-Heat Transfer

Telecurso – Ensino Médio – Física – Aula 24

Materi-Perpindahan Kalor Kelas 7 SMP
5.0 / 5 (0 votes)
