pH of Weak Acids and Bases - Percent Ionization - Ka & Kb
Summary
TLDRThis video script discusses how to calculate the pH of weak acid and base solutions, including the use of acid dissociation constant (Ka) and base dissociation constant (Kb). It covers the concepts of percent ionization and provides formulas and practice problems to help understand these calculations.
Takeaways
- 🔍 The video discusses the calculation of pH for both weak acid and weak base solutions, including the use of acid dissociation constant (Ka) and base dissociation constant (Kb).
- 📚 Reviewing basics, it's noted that strong acids like hydrochloric acid dissociate completely in water, while weak acids do not, requiring an equilibrium expression for calculation.
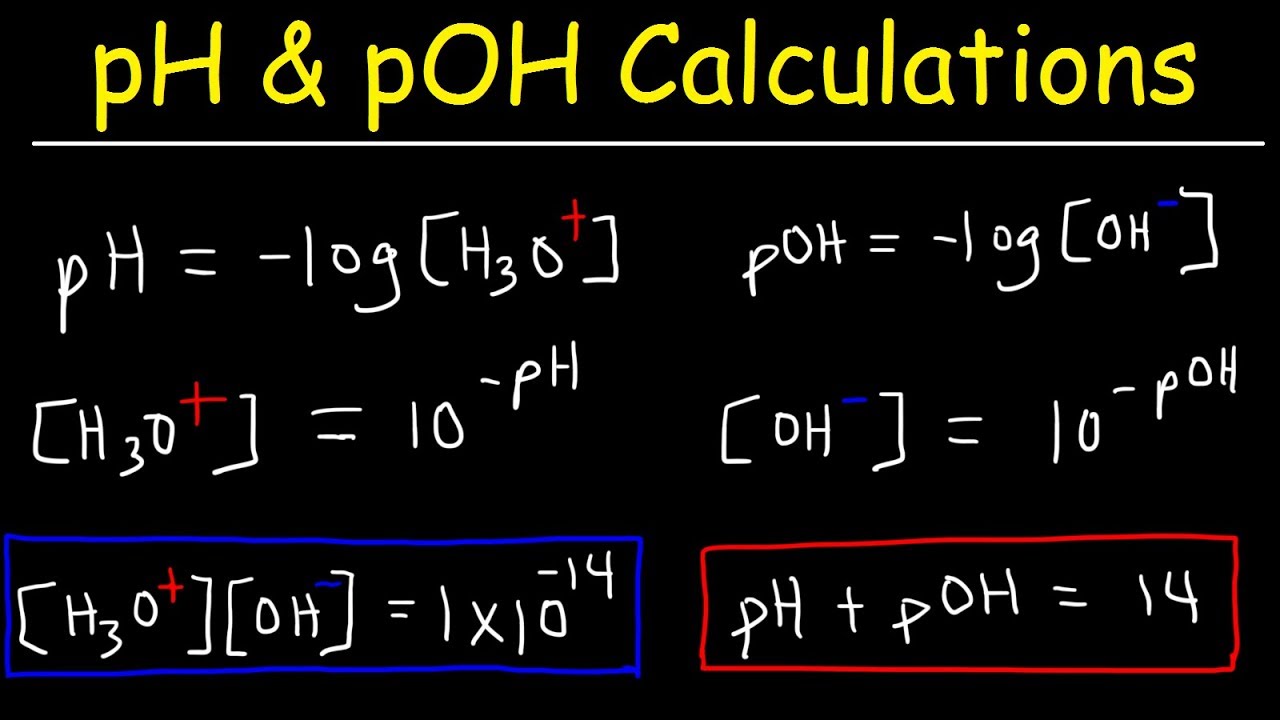
- ⚗️ The formula for calculating pH is given as pH = -log[H3O+], and for P^OH as P^OH = -log[OH-], with the relationship pH + P^OH = 14 at 25°C.
- 📉 The script explains that for weak acids, an ICE table (Initial, Change, Equilibrium) is used to calculate the concentration of H3O+ ions, which is then used to find the pH.
- 🔄 The concept of percent ionization is introduced, explaining how to calculate it for both weak acids and weak bases using the formula (x / initial concentration) * 100%.
- 🧪 Practice problems are solved in the script, demonstrating the application of these concepts to find the pH of solutions with given concentrations and Ka or Kb values.
- 🔢 The script provides a step-by-step method for weak acids, including ignoring small x values in the equilibrium expression when Ka is small, and using the quadratic formula when necessary.
- 📌 The relationship between Ka and Kb is highlighted, with the product of Ka and Kb equaling the ion product of water (Kw = 1×10^-14) at 25°C.
- 📉 The script explains how to calculate Kb from Ka, and vice versa, using the Kw value, which is essential when dealing with the pH of salt solutions derived from weak acids or bases.
- 🌡 The temperature dependence of these constants is mentioned, noting that the equations hold true specifically at 25 degrees Celsius.
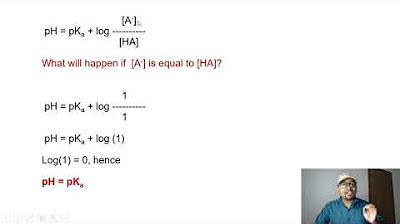
- 📝 The importance of understanding the relationship between acid strength, Ka values, and pKa is emphasized, with the stronger acid having the higher Ka value and lower pKa.
Q & A
What is the main difference between the dissociation of a strong acid and a weak acid?
-A strong acid, like hydrochloric acid, dissociates completely in water, represented by a single arrow. In contrast, a weak acid does not dissociate completely and exists in equilibrium, represented by a double arrow.
How is the pH of a strong acid solution calculated?
-The pH of a strong acid solution is calculated using the formula pH = -log[H3O+]. Since strong acids dissociate completely, the concentration of H3O+ ions is equal to the initial concentration of the acid.
What is the relationship between pH and pOH in a solution?
-The pH and pOH of a solution are related by the equation pH + pOH = 14 at 25 degrees Celsius. This is because the product of the concentrations of H3O+ and OH- ions is always 1 x 10^-14 at this temperature.
How is the acid dissociation constant (Ka) used in calculating the pH of a weak acid solution?
-Ka is used in an equilibrium expression to determine the concentration of H3O+ ions in a weak acid solution. Once H3O+ concentration is found, pH is calculated using pH = -log[H3O+].
What is the base dissociation constant (Kb) and how is it used?
-Kb is the equilibrium constant for the dissociation of a weak base. It is used to calculate the concentration of OH- ions in a weak base solution. Once OH- concentration is found, pOH is calculated, and then pH is determined by subtracting pOH from 14.
How do you calculate the concentration of H3O+ ions from pH?
-The concentration of H3O+ ions can be calculated from pH using the formula [H3O+] = 10^(-pH).
What is the relationship between Ka and Kb?
-Ka and Kb are related through the ionization constant of water (Kw), where Ka × Kb = Kw = 1 x 10^-14 at 25 degrees Celsius.
How is percent ionization calculated for a weak acid?
-Percent ionization is calculated by dividing the amount of acid that has dissociated (x) by the initial acid concentration and multiplying by 100%. In the case of weak acids, x is typically the concentration of H3O+ ions.
What is the significance of the quadratic formula in solving for x in equilibrium expressions involving weak acids or bases?
-The quadratic formula is used when the equilibrium expression cannot be simplified to ignore x. It helps solve for x in the equation ax^2 + bx + c = 0, which is derived from the equilibrium expression when x cannot be neglected.
How does the pH of a solution change when a weak base is added?
-When a weak base is added to water, it reacts reversibly to form hydroxide ions (OH-) and its conjugate acid. This increases the concentration of OH- ions, which lowers the pOH and raises the pH of the solution.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen

Ácidos/ Bases Fuertes y Débiles, Ka (Constante de disociación ácida) (1a parte)

EDUCA PE | ENSINO MÉDIO | QUÍMICA | 3º ANO | TEORIA DE ÁCIDOS E BASES E AS MEDIDAS DE PH (Parte 1)
Measurement of pKa by Potentiometry

Video Animasi Perhitungan pH Larutan Asam dan Basa
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems

Konsep Mudah belajar Hidrolisis Garam - Asam-Basa- Kimia SMA kelas 11 semester 2
5.0 / 5 (0 votes)
